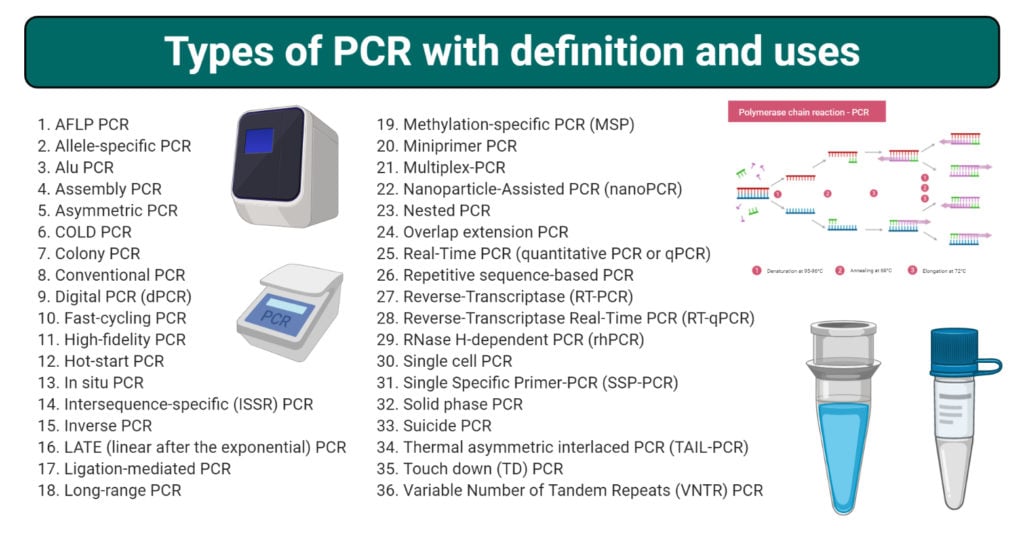
List of Types of PCR
- Amplified fragment length polymorphism (AFLP) PCR
- Allele-specific PCR
- Alu PCR
- Assembly PCR
- Asymmetric PCR
- COLD PCR
- Colony PCR
- Conventional PCR
- Digital PCR (dPCR)
- Fast-cycling PCR
- High-fidelity PCR
- High-Resolution Melt (HRM) PCR
- Hot-start PCR
- In situ PCR
- Intersequence-specific (ISSR) PCR
- Inverse PCR
- LATE (linear after the exponential) PCR
- Ligation-mediated PCR
- Long-range PCR
- Methylation-specific PCR (MSP)
- Miniprimer PCR
- Multiplex-PCR
- Nanoparticle-Assisted PCR (nanoPCR)
- Nested PCR
- Overlap extension PCR
- Real-Time PCR (quantitative PCR or qPCR)
- Repetitive sequence-based PCR
- Reverse-Transcriptase (RT-PCR)
- Reverse-Transcriptase Real-Time PCR (RT-qPCR)
- RNase H-dependent PCR (rhPCR)
- Single cell PCR
- Single Specific Primer-PCR (SSP-PCR)
- Solid phase PCR
- Suicide PCR
- Thermal asymmetric interlaced PCR (TAIL-PCR)
- Touch down (TD) PCR
- Variable Number of Tandem Repeats (VNTR) PCR
1. Amplified fragment length polymorphism (AFLP) PCR
- It is a PCR-based technique that uses selective amplification of a section of digested DNA fragments to generate unique fingerprints for genomes of interest.
- This technique can quickly generate large numbers of marker fragments for any organism, without prior knowledge of the genomic sequence.
- AFLP PCR uses restriction enzymes to digest genomic DNA and allows attachment of adaptors to the sticky ends of the fragments.
- A part of the restriction fragments is then selected to be amplified by using primers that are complementary to the adaptor sequence.
- The amplified sequences are separated and visualized on denaturing on agarose gel electrophoresis.
- AFLP PCR is employed for a variety of applications, as to assess genetic diversity within species or among closely related species, to infer population-level phylogenies and biogeographic patterns, to generate genetic maps and to determine relatedness among cultivars.
2. Allele-specific PCR
- Allele-specific polymerase chain reaction (AS-PCR) is a technique based on allele-specific primers, which can be used to analyze single nucleotide polymorphism.
- The allele-specific PCR is also called the (amplification refractory mutation system) ARMS-PCR corresponding to the use of two different primers for two different alleles.
- One is the mutant set of primers which are refractory (resistant) to the normal PCR, and the other is the normal set of primers, which are refractory to the mutant PCR reaction.
- The 3’ ends of these primers are modified such that one set of the primer can amplify the normal allele while others amplify the mutant allele.
- This mismatch allows the primer to amplify a single allele.
- It is widely applied in the single gene point mutation detection such as sickle cell anemia and thalassemia.
- It is also used for the direct determination of ABO blood group genotypes.
3. Alu PCR
- Alu PCR is a rapid and easy DNA fingerprinting technique based on the simultaneous analysis of many genomic loci surrounded by Alu repetitive elements.
- Alu elements are short stretches of DNA initially characterized by the action of the Arthrobacter luteus (Alu) restriction endonuclease.
- Alu elements are one of the most abundant transposable elements and found throughout the human genome, and they play a role in the evolution and have been used as genetic markers
- In Alu PCR, two fluorochrome-labelled primers complementary to those sequences are used to perform the PCR, and the PCR products are then analysed by
- Alu insertions have been used in several genetically inherited human diseases and various forms of cancer. Thus, this PCR plays an essential role in the detection of these diseases and mutations.
4. Assembly PCR
- Assembly PCR is a method for the assembly of large DNA oligonucleotides from multiple shorter fragments.
- In PCR, the size of oligonuleotides used is 18 base pairs, while in assembly PCR lengths of up to 50bp are used to ensure correct hybridization.
- During the PCR cycles, the oligonucleotides bind to complementary fragments and then are filled in by polymerase enzyme.
- Each cycle of this PCR thus increases the length of various fragments randomly depending on which oligonucleotides find each other.
- Assembly PCR is used to improve the yield of the desired protein and can also be used to produce large amounts of RNA for structural or biochemical studies.
5. Asymmetric PCR
- Asymmetric PCR is a variation of PCR used to preferentially amplify one strand of the original DNA more than the other.
- Asymmetric PCR differs from regular PCR by the excessive amount of primers for a chosen strand.
- As the asymmetric PCR progresses, the lower concentration limiting primer is quantitatively incorporated into newly synthesized double-stranded DNA and used up.
- Consequently, linear synthesis of the targeted single DNA strand from the excess primer is formed after depletion of the limiting primer.
- It is useful when amplification of only one of the two complementary strands is needed, such as in sequencing and hybridization probing.
6. COLD PCR
- Co-amplification at lower denaturation temperature-based polymerase chain reaction (COLD-PCR) is a novel form of PCR that selectively amplifies low-abundance DNA variants from mixtures of wild-type and mutant-containing (or variant-containing) sequences, irrespective of the mutation type or position on the amplicon.
- This method is based on the modification of the critical temperature at which mutation-containing DNA is preferentially melted over wild type.
- There is an intermediate annealing process after denaturation which allows hybridization of wild-type and mutant allele. This mismatch slightly alters the melting temperature of the ds DNA.
- These heteroduplexes will melt and will be used as a template. As s result, a more significant proportion of minor variant DNA will be amplified and be available for subsequent rounds of PCR.
- PCR plays a vital role in the detection of mutations in oncology specimens, especially in heterogeneous tumours as well as bodily fluids.
- This PCR also assists in the assessment of residual disease after surgery or chemotherapy and disease staging and molecular profiling for prognosis or tailoring therapy to individual patients.
7. Colony PCR
- Colony PCR is a method in which, where identification of DNA of interest inserted into the plasmid is obtained by designing the inserted DNA specific primers.
- The bacterial colony containing the plasmid can directly be amplified using two sets of primers.
- The first set is of the insert specific primers which amplify the insertion sequence, and the other is of vector-specific flanking primers, which amplifies the plasmid DNA other than the inserted DNA.
- A bacterial colony is taken and added directly into the master mix containing all other PCR reagents.
- The main application of colony PCR is in the identification of correct ligation and insertion of inserted DNA into bacteria as well as yeast plasmid.
8. Conventional PCR
- The polymerase chain reaction(PCR) is a test tube system for DNA replication which allows a “target” DNA sequence to be selectively amplified several million folds in just a few hours.
- PCR enables the synthesis of specific DNA fragments using a DNA-polymerase enzyme, which takes part in the replication of the cellular genetic material.
- This enzyme synthesizes a complementary sequence of DNA, as a small fragment (primer) is connected to one of the DNA strands in the specific site chosen to start the synthesis.
- Primers limit the sequence to be replicated, and the result is the amplification of a particular DNA sequence with billions of copies.
- Conventional PCR is applied in selective DNA isolation, amplification and quantification of DNA, medical and diagnostic approaches, infectious disease diagnosis, forensic studies and research areas.
9. Digital PCR (dPCR)
- Digital PCR (dPCR) is a quantitative PCR technology that provides a sensitive and efficient way for the measurement of the amount of DNA or RNA present in a sample.
- For dPCR, the initial sample mix is divided into a large number of individual wells prior to the amplification step, resulting in either target sequence being present in each well or not.
- Based on the presence or absence of fluorescence in the amplified reaction wells calculation of the absolute number of targets present in the original sample is done.
- Wells with a fluorescent signal are considered positives and scored as “1” while wells with no such signal are negatives and scored as “0”.
- The concentration of the target sequence present in the initial sample is then determined through Poisson statistical analysis.
- dPCR is used to determine the total numbers of DNA and RNA viruses, bacteria, and parasites in a variety of clinical specimens, mainly when a well-calibrated standard is not available.
10. Fast cycling PCR
- Fast cycling PCR is a PCR-based technology that allows amplification of specific PCR products with significantly reduced cycling time.
- The principle in this process is the same as conventional PCR, the only difference being the time of amplification.
- The buffer used in this PCR increases the affinity of Taq DNA polymerases for short single-stranded DNA fragments, reducing the time required for successful primer annealing to just 5 seconds.
- Fast cycling PCR is essential for processes requiring quick cycles and also helps in the rapid diagnosis of diseases and mutations.
11. High Fidelity PCR
- High-fidelity PCR is a modifies PCR method that utilizes a DNA polymerase with a low error rate and results in a high degree of accuracy in the replication of the DNA of interest.
- Such enzymes have a significant binding affinity for the correct nucleoside triphosphate during amplification.
- In the case of an incorrect binding in the polymerase active site, incorporation is slowed due to the architecture of the active site complex.
- Highfidelity amplification is essential for experiments whose outcome depends upon the correct DNA sequence like cloning, SNP analysis, NGS applications.
12. High-Resolution Melt (HRM) PCR
- It is a hugely powerful technique for the detection of mutations, polymorphisms and epigenetic differences in double stranded DNAsamples.
- It is massively cost-effective vs. other genotyping technologies such as sequencing and Taqman SNP typing. This makes it ideal for large scale genotyping projects.
- It is fast and powerful thus able to accurately genotype huge numbers of samples in rapid time.
- It is simple. With a good quality HRM assay powerful genotyping can be performed by non-geneticists in any laboratory with access to an HRM capable real-time PCR machine.
13. Hot start PCR
- Hot start PCRis a novel form of conventional polymerase chain reaction (PCR) that reduces the occurrence of undesired products and formation of primer-dimers due to non-specific DNA amplification at room temperatures.
- The basic principle of hot-start PCR is the separation of one or more reagents from the reaction mix until the mixture reaches the denaturation temperature upon heating.
- Hot start PCR significantly reduces non-specific binding, the formation of primer-dimers, and often increases product yields. It also requires less effort and reduces the risk of contamination.
14. In-situ PCR
- In-Situ Polymerase Chain Reaction(In-situ PCR) is an effective method that detects minute quantities of rare nucleic acid sequences in frozen or paraffin-embedded cells or tissue sections for the compartmentalization of those sequences within the cells.
- This method involves tissue fixing that preserves the cell morphology, which is then followed by the treatment with proteolytic enzymes to provide an entry for the PCR reagents to act on the target DNA.
- The target sequences are amplified by the reagents and then detected by standard immunocytochemical protocols.
- In-situ PCR is applicable for the diagnosis of infectious diseases, quantification of DNA, detection of even small amount of DNA and is widely used in the study of organogenesis and embryogenesis.
15. Intersequence specific (ISS) PCR
- InterSequence-Specific PCR (or ISSR-PCR) is a method for DNA fingerprinting that uses primers selected from specific segments repeated throughout a genome to produce a unique fingerprint.
- The technique uses microsatellites, usually 16–25 bp long, as primers in a single primer PCR reaction targeting multiple genomic loci to amplify mainly the inter- SSR sequences of different sizes.
- ISSR PCR can be used in genomic fingerprinting, genetic diversity and phylogenetic analysis, genome mapping and gene tagging.
16. Inverse PCR
- Inverse polymerase chain reaction (Inverse PCR) is one of the many variants of the polymerase chain reaction that is used to amplify DNA when only one sequence is known.
- Conventional PCR requires primers complementary to both terminals of the target DNA, but Inverse PCR allows amplification to be carried out even if only one sequence is available from which primers may be designed.
- The inverse PCR involves a series of restriction digestion followed by ligation, which results in a looped fragment that can then be primed for PCR through a single section of known sequence.
- Then, like other polymerase chain reaction processes, the DNA is amplified by the temperature-sensitive DNA polymerase.
- Inverse PCR is especially useful for the determination of insert locations of various transposons and retroviruses in the host DNA.
17. LATE (Linear-After-The-Exponential) PCR
- LATE (Linear-After-The-Exponential) PCR is a modification of Asymmetric PCR which uses a limiting primer with a higher melting temperature than the excess primer which maintains reaction efficiency as the limiting primer concentration decreases mid-reaction.
- LATE-PCR begins with an exponential phase in which amplification efficiency is similar to that in conventional PCR. Once the limiting primer is depleted, the reaction abruptly switches to linear amplification, and the single-stranded product is continued for many additional thermal cycles.
18. Ligation mediated PCR
- Ligation-mediated PCR is a modified form of conventional PCR that is possible with the knowledge of only one end initially and then adding the second end by ligation of a unique DNA linker.
- Ligation-mediated PCR utilizes small DNA fragments called ‘linkers’ (or adaptors) that are initially ligated to fragments of the target DNA.
- PCR primers designed to bind to the linker sequences are then used to amplify the target fragments.
- This method is deployed for DNA sequencing, genome walking, and DNA footprinting.
19. Long-Range PCR
- Long-Range PCR is a method for the amplification of longer DNA lengths that cannot typically be amplified using routine PCR methods or reagents.
- Long-range PCR can be achieved by using modified high-efficiency polymerases with enhanced DNA binding, resulting in highly processive and accurate amplification of long fragments.
- This method allows the amplification of more extended targets within a shorter period and with efficient use of resources.
20. Methylation-specific PCR (MSP)
- Methylation-specific PCR (MSP) is a method for the detection and analysis of DNA methylation patterns in CpG islands.
- For performing MSP, DNA is modified by, and PCR performed with two primer pairs, which are detectable methylated and unmethylated DNA, respectively.
- The DNA undergoes treatment with bisulfite for the conversion of cytosine to uracil, and then the methylated sequences are selectively amplified with primers specific for
- Detection of methylated patterns is essential as excessive methylation of CpG dinucleotides in promoter represses the gene expression.
21. Miniprimer PCR
- A new PCR method using an engineered polymerase and 10-nucleotide “miniprimers” is termed Miniprimer PCR.
- This method is found to reveal novel 16S rRNA gene sequences that would not have been detected with standard primers.
- Miniprimer PCR uses a thermostable polymerase enzyme that can extend from short primers (9 or 10 nucleotides).
- This method allows PCR targeting to smaller primer binding regions, and is used to amplify highly conserved DNA sequences, such as the 16S (or eukaryotic 18S) rRNA gene.
22. Multiplex PCR
- Multiplex PCR is a common molecular biology technique used for the amplification of multiple targets in a single PCR test run.
- In Multiplex PCR, multiple primers and a temperature-mediated DNA polymerase are used for the amplification of DNA in a thermal cycler.
- All the primers pairs designed for Multiplex PCR have to be optimized so that the same annealing temperature is optimal for all the pairs during PCR.
- When multiple sequences are targeted at once, additional information can be generated from a single test run which otherwise would require a larger amount of the reagents and extensive time and effort to perform.
- This technology has been applied in many areas such as genotyping, mutation and polymorphism analysis, microsatellite STR analysis, detection of pathogens or genetically modified organisms, etc.
- In diagnostic laboratories, multiplex PCR is useful to detect different microorganisms that cause the same types of diseases.
23. Nanoparticle-Assisted PCR (nanoPCR)
- A nanoparticle associated PCR includes small molecular substances comprising of particular physical properties that enhance the reaction.
- One of the theories involving the gold nanoparticles states that these particles adsorb some of the polymerase and manages the amount of polymerase remaining in the system, which might be necessary in enhancing the specificity of the reaction.
- Another theory explains that they adsorb primer pairs and lower the melting temperature at duplex formation between perfectly paired and mispaired primers, which leads to an increase in the specificity of the reaction.
- Nanoparticle associated PCR has advantages of high sensitivity, high specificity and high selectivity, and has been widely used in virus detection and gene sequencing.
24. Nested PCR
- Nested PCR is a useful modification of PCR technology where the specificity of the reaction is enhanced by preventing the non-specific binding with the help of the two sets of primer.
- The first set of primer binds outside of our target DNA and amplifies larger fragment while another set of primer binds specifically at the target site.
- In the second round of amplification, second set of primer amplifies only the target DNA.
- Nested PCR is a helpful method for the phylogenetic studies and detection of different pathogens.
- The technique has higher sensitivity; hence even if the sample contains lower DNA, it can be amplified which is not feasible in the conventional PCR technique.
25. Overlap extension PCR (OE-PCR)
- This method is also called “Splicing by Overlap Extension” or SOEing.
- Overlap extension PCR is a valuable technique that is commonly used for cloning large complex fragments, making edits to cloned genes or fusing two gene elements together.
- It creates long DNA fragments from shorter ones.
- It is used for efficient gene cloning and multiple site-directed large fragments insertion, deletion and replacement.
- It is proven useful for site-directed mutagenesis, the creation of chimeric molecules or even the cloning of large gene segments by splicing together smaller pieces.
26. Real-Time PCR (Quantitative PCR (qPCR))
- Quantitative PCR (qPCR), also called real-time PCR or quantitative real-time PCR, is a PCR-based technique that couples amplification of a target DNA sequence with quantification of the concentration of that DNA species in the reaction.
- Conventional PCR is a time-consuming process where the PCR products are analysed through gel electrophoresis. qPCR facilitates the analysis by providing real time detection of products during the exponential phase.
- The principle of real-time PCR depends on the use of fluorescent dye.
- The concentration of the nucleic acid present into the sample is quantified using the fluorescent dye or using the fluorescent labelled oligonucleotides.
- q-PCR is applied in genotyping and quantification of pathogens, microRNA analysis, cancer detection, microbial load testing and GMOs detection.
27. Repetitive sequence-based PCR
- Repetitive sequence-based PCR (rep-PCR) is a modified PCR technology that uses primers that target noncoding repetitive sequences interspersed throughout the bacterial genome.
- Such blocks of noncoding, repetitive sequences can serve as multiple genetic targets for oligonucleotide probes, enabling the generation of unique DNA profiles or fingerprints for individual bacterial strains.
- The main application of rep-PCR is in the molecular strain typing of different bacteria. It is also used for epidemiologic discrimination of various pathogens.
28. Reverse Transcriptase PCR (RT-PCR)
- Reverse transcription PCR (RT-PCR) is a modification of conventional PCR, whereby RNA molecules are first converted into complementary DNA (cDNA) molecules that can then be amplified by PCR.
- In RT-PCR, the RNA template is first converted into a complementary DNA (cDNA) using reverse transcriptase. The cDNA then acts as a template for exponential amplification using PCR.
- RT-PCR can be conducted either in a single tube or as two steps in different tubes. The one-step method is more effective with fewer chances of contamination and incorporation of variations.
- RT-PCR is used in research methods, gene insertion, genetic disease diagnosis and cancer detection.
29. Reverse-Transcriptase Real-Time PCR (RT-qPCR)
- RT-PCR is commonly associated with q-PCR forming Reverse Transcriptase Real-Time PCR (RT-qPCR).
- This allows quantification of DNA in real-time after the amplification.
30. RNase H-dependent PCR
- In the RNase H-dependent PCR, the primers contain a removable amplification block on their 3’ end.
- The blocked primer can only perform amplification depending on the cleavage activity of an RNase Henzyme during hybridization to the complementary target sequence.
- RNase H enzyme has very little enzymatic activity at a low temperature, enables a hot start without any modification to the DNA polymerase.
- Similarly, the cleavage efficiency of the enzyme is reduced in the presence of mismatches near the RNA residue.
- Thus, under the activity of the RNase H enzyme, the non-specific binding and primer dimer formation is reduced, enabling effective hybridization.
31. Single Specific Primer PCR
- The single specific primer-PCR (SSP-PCR) is a PCR-based technology that permits amplification of genes of which, only a piece of partial sequence information is available.
- It allows unidirectional genome walking from known into unknown regions of the chromosome.
32. Single Specific Primer-PCR (SSP-PCR)
- This allows the amplification of double-stranded DNA even when the sequence information is available at one end only.
- This method, the single specific primer-PCR (SSP-PCR), permits amplification of genes for which only a partial sequence information is available, and allows unidirectional genome walking from known into unknown regions of the chromosome.
33. Solid Phase PCR
- Solid-phase PCR (SP-PCR) is a unique PCR technique that allows amplification of target nucleic acids on a solid support where one or both primers are immobilized on the surface.
- The spatial separation of the primers minimizes significantly undesirable primer interactions, thereby preventing the formation of primer-dimers and allowing higher multiplexing amplification.
- The central idea of this novel method is to attach the 5′-end of the primers to a surface instead of letting the primers freely diffuse in a bulk solution.
- A freely diffusing DNA target can be captured on the surface and then copied by the polymerase.
- The copy stays attached to the surface, whereas the initial DNA molecule returns to the solution after the annealing step.
- The free end of the attached copy hybridizes to the primer (attached to the surface) complementary to its sequence, and the amplification process can start.
34. Suicide PCR
- Suicide PCR is commonly used studies where avoiding false positives and ensuring the specificity of the amplified fragment is the highest priority.
- The method requires the use of any primer combination only once in a PCR, which should not have been used in any positive control PCR reaction.
- These primers should always target a genomic region which has never been amplified before using this particular primer or any other set of primers.
- This arrangement ensures that no contaminating DNA from previous PCR reactions is present in the lab, which could otherwise generate false positives.
- Suicide PCR is used in paleogenetics studies which involve an examination of preserved genetic material from the remains of ancient organisms.
35. Thermal asymmetric interlaced PCR (TAIL-PCR)
- TAIL PCR is a powerful tool for the recovery of DNA fragments adjacent to known sequences.
- TAIL –PCR utilizes three nested primers in consecutive reactions together with an arbitrary degenerate primer having a low melting temperature so that relative amplification frequencies of specific and non-specific products can be thermally controlled.
- This method is highly accurate such that the unpurified TAIL-PCR products can be directly sequenced.
- It also allows the cloning of full-length functional genes.
36. Touch down PCR
- Touch Down PCR is a modification of PCR in which the initial annealing temperature is higher than the optimal Tm of the primers and is gradually reduced over subsequent cycles until the Tm temperature or “touchdown temperature” is reached.
- Touchdown PCR increases the specificity of the reaction at higher temperatures and increases the efficiency towards the end by lowering the annealing temperature.
37. Variable Number of Tandem Repeats (VNTR) PCR
- They are important markers for the individualization in forensic science.
- In VNTR PCR, fragments are amplified that showed little variation within a species, but did show differences between species.
- It can successfully amplify from a very small amount of genomic deoxyribonucleic acid (DNA) by the polymerase chain reaction (PCR).
- Among the genotyping tools, the PCR-based variable-number tandem repeat (VNTR) analysis represented a promising method for typing M. tuberculosis.








0 Comments