Bacillus anthracis definition
Bacillus anthracis is the only obligate pathogenic species of Bacillus and the causative agent of anthrax, which is a common disease of livestock and occasionally occurs in humans.
- Anthrax is called a zoonotic disease as the disease can transfer between animals and humans via different means.
- It is a Gram-positive spore-forming bacterium that is commonly found in soil, but unlike other Bacillus species, can result in different forms of infections if it reaches the respiratory, gastrointestinal, or cutaneous regions in humans.
- The most important mode of transmission of B. anthracis is through spores. These spores germinate into vegetative cells once inside the body of the host and cause infections.
- The spores are highly resistant to adverse environmental conditions, but the vegetative cells of B. anthracis survive poorly in simple environments like water and bulk soil.
- B. anthracis belongs to the B. cereus group of Bacillus species, consisting of other opportunistic pathogens like B. cereus and B. thuringiensis.
- The potential of B. anthracis as a bioweapon or a bioterrorism agent has been discussed and feared for a long time after the use of this bacterium against livestock during World War I.
- However, it has been found that humans are more resistant to B. anthracis than herbivores, and the infectious doses of the bacterium in humans are also very high.
- Nevertheless, human anthrax cases have been observed in the human population through close occupational proximity to infected livestock by handling infected domestic animals.
- B. anthracis was first isolated from infected domestic animals by Cohn in 1872.
- The species name ‘anthracis’ is derived from the name of the disease, anthrax, which in turn is derived from the Greek word for coal, anthrakis due to the formation of black, coal-like cutaneous eschars.
- B. anthracis has been studied extensively due to the pathogenicity of the species and its potential as a bioterrorism agent.
Classification of Bacillus anthracis
- The genus Bacillus belongs to the family Bacillaceae which contains other several genera classified on the basis of their phenotypic and molecular characteristics.
- There are more than 142 different species belonging to the genus Bacillus which are further classified into manageable groups on the basis of their 16S rRNA sequences.
- B. anthracis belongs to the B. cereus group of Bacillus species along with other pathogens or opportunistic pathogens like B. cereus and B. thuringiensis.
- The genomes of the group are highly conserved with sizes of 5.2-5.5 Mb and also have very similar 16S rRNA gene sequences.
- The classification and distinction of B. anthracis from other members of the group can be made by amplified fragment length polymorphism analysis as they share similar 16S rRNA gene sequences.
- There are 89 different strains of B. anthracis that have been isolated from different areas throughout the world and are used for different purposes.
- B. anthracis is monomorphic and has low genetic diversity along with the absence of any measurable lateral DNA transfer.
- The strains of this species are genetically and phenotypically heterogeneous overall, but some of the strains are more closely related than others as some are phylogenetically intermixed at the chromosome level.
- Some differences, however, might be observed as the lifecycle of the bacteria revolves between an animal host and the environment.
The following is the taxonomical classification of B. anthracis:
| Domain | Bacteria |
| Phylum | Firmicutes |
| Class | Bacilli |
| Order | Bacillales |
| Family | Bacillaceae |
| Genus | Bacillus |
| Species | B. anthracis |
Habitat of Bacillus anthracis
- Bacillus anthracis is an aerobic endospore-forming bacterium that exists in a wide range of natural environments or ecosystems.
- It is the only obligate pathogen of animals, including humans, mammals, and insects. The main habitat, however, remains the soil and it might transmit to other ecosystems in the form of spores.
- Even though the bacteria have been isolated from different environments, all of those environments are not considered their habitats.
- B. anthracis, like other Bacillus species, thrives in the soil of both acidic and alkaline conditions within a wide temperature range.
- B. anthracis, in the natural environment, outside the host body, exists in the form of spores as the environmental condition gets difficult.
- The habitat and lifecycle of B. anthracis are described by the micro cycling of the bacteria in soil with infrequent multiplication phases in animals.
- Even though there is not much evidence on the multiplication of B. anthracis in soils, it is believed that the spores of B. anthracis can germinate into the vegetative cell and undergo multiplication if the environmental conditions are favorable.
- The ability of B. anthracis to exist in such diverse environments is the result of highly resistant endospores. These endospores are more resistant to adverse conditions, some chemicals, and even antimicrobial agents than vegetative cells.
- The spores are generally light and can be easily distributed through dust or the formation of aerosols.
- The spores thus, enter the body of the host (mostly herbivore) where they germinate to form vegetative cells. The vegetative cells then might enter new niches like the human body from the animals.
- Besides, there are other mechanisms like toxin production, which enables the bacteria to compete against other bacteria and occupy new ecology or niches.
- The occurrence of B. anthracis is more common in warmer climates, which is assumed to be due to the relationship between temperature and water activity, and rates of sporulation of the bacteria shed from the body of the infected animal.
- Similarly, temperature and water activity also influence the germination of spores, although to a lesser extent.
- One of the essential features for the occurrence of B. anthracis in diverse environments is the ability of the spores to survive for many years, even in the absence of animal reservoirs.
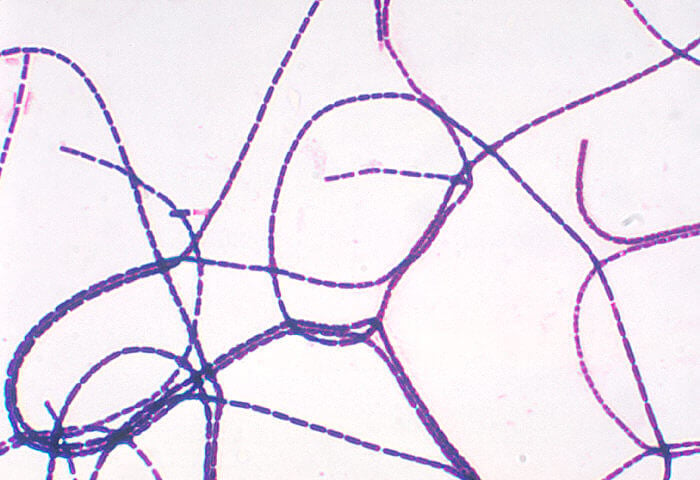
Figure: Gram-positive, rod-shaped, Bacillus anthracis bacteria. Image Source: CDC/ Dr. James Feeley.
Morphology of Bacillus anthracis
- The cells of B. anthracis are Gram-positive rods that are aerobic, facultative anaerobes that are capsulated and can form spores.
- The cells of the size ranging from 1.0-1.2 µm in width and 3.0-5.0 µm in length occur either singly or in pairs. In the clinical samples, however, cells might appear in short chains.
- All the cells of B. anthracis contain ellipsoidal or cylindrical spores that are present either subterminally or paracentrally in the vegetative cell.
- The spores do not result in the swelling of the sporangia as they usually lie obliquely in it.
- Phenotypically, B. anthracis is very similar to other Bacillus species like B. cereus and B. thuringiensis but, unlike them, B. anthracis does not have flagella and thus, is non-motile.
- The outer covering of B. anthracis is defined by a capsule, extensive peptidoglycan layer, lipoteichoic acids, and crystalline cell surface proteins (S layer).
- The capsule in B. anthracis is composed of poly-γ-D-glutamic acid encoded by three different plasmid genes.
- The capsule of B. anthracis is one of the virulence factors as the strains that do not have the capsule are avirulent.
- The capsule in itself is non-toxic and non-immunogenic as it doesn’t stimulate the host immune system.
- Underneath the capsule is the surface layer or S-layer composed of proteins, and these are not glycosylated.
- The cell wall polysaccharides present on the cell wall function in anchoring the surface layer to the cell wall. The cell wall is composed of polysaccharides like galactose, N-acetylglucosamine, and N-acetylmuramic acid.
- The linkages present in the cell wall are of the meso-diaminopimelic acid which connects the diamino acid of one subunit to the D-alanine group of another subunit.
- The genome of B. anthracis is tripartite with a single circular chromosome and two circular virulent plasmids in the cytoplasm. The genome is 5227293 bp long with 5508 protein-coding sequences.
- The genome nucleotide composed is composed of about 60% adenine and thymine units and just 40% of guanine and cytosine.
- This composition of the chromosome results in higher buoyant density and a lower melting point of the DNA units.
- The plasmids are pXO1 and pXO2 that code for many different genes along with toxin and capsule production genes.
Cultural Characteristics of Bacillus anthracis
- The artificial growth and culture of B. anthracis are often done by the isolation of the bacteria from old carcasses, animal products, or environmental samples like soil where they exist in the form of spores.
- The isolation of B. anthracis can be achieved on blood, nutrient, or selective agars, depending on the sample source.
- There is no efficient enrichment method for the isolation of B. anthracis, but considerable selective isolation can be done with polymyxin-lysozyme EDTA-thallous acetate (PLET) agar.
- Like most Bacillus species, the nutrient requirement of B. anthracis is also simple, thus growth can be achieved in simple media with glucose as a source of carbon and an ammonium salt as the source of nitrogen.
- Hydrolyzed casein medium with glucose, thiamine, tryptophan, and various salts is usually used for the analysis of B. anthracis physiology and gene expression.
- The colony morphology, as well as growth, is influence by various factors like the germination of spores, medium composition, and incubation conditions.
- Heat treatment of spores prior to growth stimulates the germination of spores and the formation of vegetative cells.
- B. anthracis is a facultative anaerobe; thus, the best growth is observed when incubated overnight under 5-7% CO2.
- Colony morphology and the surface of the colonies also depend on the presence or absence of capsules as capsulated colonies form mucoid colonies.
- The growth of B. anthracis can occur between 5°C to 45°C with optimum growth at 37°C, depending on the source of bacteria.
- The colonies of B. anthracis are similar to other species of Bacillus and can be distinguished on the basis of spiking or tailing along the lines of inoculation streaks and are very tenacious.
- The growth of B. anthracis might occur in a swarming fashion all over the media instead of individual colonies. This can be avoided by increasing the agar content of the medium.
- In the case of liquid media, B. anthracis generally grows as planktonic cells, but pellicles can form during static incubation, and adherence to solid surfaces might also be observed.
- The growth of B. anthracis on an artificial medium occurs in two phases beginning with vegetative growth leading to spore formation as the culture gets older.
The following are some cultural characteristics of B. anthracis on different culture media:
1. Bacillus anthracis on Nutrient Agar (NA)
- Depending on the sample, B. anthracis can be grown on different types of growth medium from a simple medium like Nutrient agar to a more complex and selective medium like PLET agar.
- The colonies of B. anthracis are similar to that of other members of the B. cereus group and might require an additional technique for species-level identification.
- On NA, colonies of B. anthracis appear circular to irregular with entire to undulate margins and crenate or fimbriate edges.
- When the growth conditions are not conducive for capsule formation, the colonies have irregular edges and a round ‘ground-glass’ appearance.
- The colonies are white to cream-colored with large sizes (2-7 mm in diameter), but the size of young colonies can be smaller.
- Colonies of B. anthracis are different from other Bacillus species in that they form spiking or tailing along the lines of inoculation. Some colonies might even form standing peaks when pulled with a loop.
- The surface of the colonies tends to have matt or granular textures, but smooth and moist colonies might also occur.
- Colonies on solid media favor capsule synthesis which results in mucoid colonies with a large capsule, resulting in thick colonies (up to 3µm in thickness).
2. Bacillus anthracis on Blood Agar
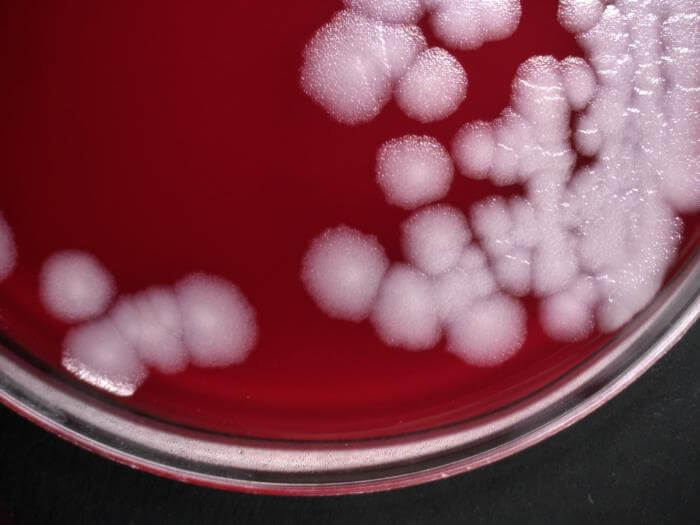
Figure: Bacillus anthracis, which were cultured on sheep blood agar (SBA) medium, for a 24-hour time period, at a temperature of 37°C. Image Credit: Todd Parker
- The blood agar used for the isolation of B. anthracis is prepared by adding 5% sheep blood to nutrient agar.
- The colonies of B. anthracis are non-hemolytic or γ-hemolytic, which helps in the differentiation of B. anthracis from other Bacillus species like B. cereus and B. thuringiensis.
- Flat or slightly convex colonies with irregular edges and ground glass appearance are observed. The colonies often have comma-shaped projections from the edge of the colonies, resulting in medusa-head colonies.
- The size of the colonies is comparatively smaller than that on Nutrient agar. The size ranges between 2-4 mm, but the size might increase on the second day of growth.
3. Bacillus anthracis on PLET Agar
- PLET agar is the best selective agar for the isolation of B. anthracis from environmental specimens, animal products as well as clinical specimens.
- The increased concentration of EDTA on this media inhibits the growth of Staphylococcus aureus as well as B. cereus.
- The colonies of B. anthracis are roughly circular, creamy-white with a glass texture. If the colonies of B. cereus are seen, they tend to be smaller than that of B. anthracis.
- The capsule might be visible on the surface after about 48 hours of incubation.
Figure: Bacillus anthracis cultured on mannitol, egg yolk, polymyxin agar (MEP) medium, for a 24-hour time period, at a temperature of 37°C. Image Credit: Todd Parker
Biochemical Characteristics of Bacillus anthracis
The biochemical characteristics of B. anthracis can be tabulated as follows:
| S.N | Biochemical Characteristics | B. anthracis |
| 1. | Capsule | Capsulated with a poly-γ-glutamic acid capsule. |
| 2. | Shape | Rod |
| 3. | Gram Staining | Gram-Positive |
| 4. | Catalase | Positive (+) |
| 5. | Oxidase | Negative (-) |
| 6. | Citrate | Positive (+) |
| 7. | Methyl Red (MR) | Negative (-) |
| 8. | Voges Proskauer (VR) | Positive (+) |
| 9. | OF (Oxidative-Fermentative) | Facultative Heterofermentative |
| 10. | Coagulase | Positive (+) |
| 11. | DNase | Negative (-) |
| 12. | Urease | Negative (-) |
| 13. | Gas | Negative (-) |
| 14. | H2S | Negative (-) |
| 15. | Hemolysis | Non-hemolytic |
| 16. | Motility | Non-motile as they lack flagella. |
| 17. | Nitrate Reduction | Positive (+) |
| 18. | Gelatin Hydrolysis | Positive (+) |
| 19. | Pigment Production | Negative (-) |
| 20. | Indole | Positive (+) |
| 21. | TSIA (Triple Sugar Iron Agar) | Alkali/Alkali (Red/ Red) |
| 22. | Spore | Endospore-forming |
| 23. | Penicillin Susceptibility | Susceptible |
Fermentation
| S.N | Substrate | B. anthracis |
| 1. | Adonitol | Negative (-) |
| 2. | Arabinose | Negative (-) |
| 3. | Cellobiose | Negative (-) |
| 4. | Dulcitol | Negative (-) |
| 5. | Fructose | Positive (+) |
| 6. | Galactose | Negative (-) |
| 7. | Glucose | Positive (+) Facultative heterofermentative |
| 8. | Glycerol | Negative (-) |
| 9. | Glycogen | Positive (+) |
| 10. | Hippurate | Negative (-) |
| 11. | Inulin | Negative (-) |
| 12. | Inositol | Negative (-) |
| 13. | Lactose | Negative (-) |
| 14. | Malonate | Positive (+) |
| 15. | Maltose | Positive (+) |
| 16. | Mannitol | Negative (-) |
| 17. | Mannose | Positive (+) |
| 18. | Melibiose | Negative (-) |
| 19. | Pyruvate | Negative (-) |
| 20. | Raffinose | Negative (-) |
| 21. | Rhamnose | Negative (-) |
| 22. | Ribose | Positive (+) |
| 23. | Salicin | Negative (-) |
| 24. | Sorbitol | Negative (-) |
| 25. | Starch | Positive (+) |
| 26. | Sucrose | Positive (+) |
| 27. | Trehalose | Positive (+) |
| 28 | Xylose | Negative (-) |
Enzymatic Reactions
| S.N | Enzymes | B. anthracis |
| 1. | Acetoin | Positive (+) |
| 2. | Acetate Utilization | Positive (+) |
| 3. | Tyrosine Hydrolysis | Negative (-) |
| 4. | Lecithinase | Negative (-) |
| 5. | Casein Hydrolysis | Positive (+) |
| 6. | Esculin Hydrolysis | Positive (+) |
| 7. | Lysine decarboxylase | Positive (+) |
| 8. | Ornithine Decarboxylase | Negative (-) |
| 9. | Phenylalanine Deaminase | Negative (-) |
Virulence Factors of Bacillus anthracis
Bacillus anthracis is an obligate pathogenic bacterium that is the causative agent of the disease, anthrax, occurring commonly in herbivores and less often in humans. However, animals like pigs, dogs, cats, rats, and chickens are resistant to anthrax, but the bacteria can transfer to birds after scavenging birds like vultures feed on infected dead animals. The most important factor that enables the bacteria to survive and cause infections is the ability of the bacteria to form spores. The spores are resistant against different adverse environmental conditions. Besides the three most important virulence factors of B. anthracis, there are other secreted and non-secreted factors that affect host-pathogen interactions. There are certain proteases that interfere with the immune response by cleaving the antimicrobial peptides. Other proteases like InhA1 can degrade host tissues, resulting in increased barrier permeability. B. anthracis has extraordinary virulence in the form of three primary virulence factors;
1. Capsule
- B. anthracis produces a poly-γ-glutamic acid (PGA) capsule that provides protection of the bacterium from phagocytosis as in many other pathogenic bacteria.
- The presence of a negative charge on the membrane inhibits host defense by inhibiting phagocytosis of the vegetative cells by macrophages and other immune cells.
- The capsule is produced by spores germinating in the presence of serum and elevated CO2 through openings on the spore surface in the form of pore which may coalesce before sloughing of the exosporium and outgrowth of the encapsulated vegetative cells.
- As the capsule is present exterior to the S layer, it does not require the S-layer for its attachment to the cell surface.
- The synthesis of the capsule is facilitated by three membrane-associated enzymes encoded by the 60-MDa pXO2 plasmid.
- The genes involved in the enzyme synthesis are capB, capC, and capA encoding for 44, 16, and 46kDa proteins, respectively.
- The capsule of B. anthracis is weakly immunogenic and antiphagocytic that disguises the bacilli from immune surveillance.
- The capsule also activates caspase-1 and induces the release of interleukin-1β from differentiated T-cells and human monocyte-derived dendritic cells.
- The activity of capsule proteins is enhanced by other antigens, proteins, and plasmid-encoded toxins.
2. Endotoxin
- Bacillus anthracis produces two different endotoxins which are released in the form of three components; protective antigen (PA), edema factor (EF), and lethal factor (LF).
- All of the three proteins are encoded by the virulence plasmid pXO1 which causes hemorrhage, edema, and necrosis.
- The protective antigen is the cellular binding part of the toxins while the lethal factor and edema factor are the catalytic parts.
- The edema factor is a mature protein with 767 residues with a molecular mass of 89 kDa which is a calmodulin-dependent adenylate cyclase converting intracellular ATP into cAMP.
- The amino terminal part of the factor is expressed as a stable polypeptide that has the capacity to compete with LF for binding to the protective antigen.
- The lethal factor is also a mature protein with 776 resides and the molecular mass of 85kDa, which is a zinc metalloprotease that cleaves and inactivates the nitrogen-activated protein kinase.
- Like in the edema factor, a lethal factor also has an aminoterminal part that enables the binding of the molecule to PA.
- The PA following its release is proteolytically cleaved by furin or furin-like proteases into two fragments; PA63 and PA20. The removal of PA20 removes the steric hindrance and allows PA63 to form an LF/EF-binding component.
- The cleavage results in residues on the PA63, which can bind 3 or 4 EF and LF molecules while maintaining steric hindrance interference between the toxin molecules. The binding eventually leads to the formation of lethal toxin (LT) and edema toxin (ET).
- The toxins play a crucial role in responses to a diverse array of stimuli like mitogens, proinflammatory cytokines, and heat shock.
Read Also: 29 Difference between exotoxins and endotoxins
Pathogenesis of Bacillus anthracis
The cycle of infection of B. anthracis begins with the ingestion of spores, which in the case of animals occurs from the soil while in humans, it is transmitted from the animals after frequent exposure. The extraordinary virulence of the bacteria is due to the virulence factors that enable the survival of the bacteria as well as its ability to cause destruction of the host cell during its infectious life cycle. The overall pathogenesis of B. anthracis can be described in the following steps;
1. Entry
- The primary infectious form of B. anthracis is the spore that enters the body of the host from the environment by different means.
- The spores are resistant to various environmental conditions and can germinate into vegetative form as the favorable condition becomes available.
- Macrophages rapidly phagocytose the spores inside the host’s body, and some of the spores undergo lysis in the macrophages.
- Other spores, especially those that enter the body via inhalation, survive the phagocytosis and are carried towards the mediastinal lymph nodes by the lymphatic system.
- The phagocytosed spores require some days of incubation before germination. This latency is observed in the respiratory form of the disease and not in the cutaneous form.
- The germination is triggered by elevated CO2 levels and the body temperature of the host.
2. Invasion
- The germination of the spores into vegetative cells is followed by the activation of capsule and toxin genes present in the plasmids of the organism.
- The capsule is involved in the resistance to phagocytosis as a result of negative charges present on it.
- The toxins are also released in the form of three different protein components that undergo cleavage and binding to form the toxins eventually.
- The protective antigen (PA) binds to the molecules of a particular host cell membrane protein which in this case is the anthrax toxin receptor (ATR).
- PA is then cleaved by proteases into two parts, one of which binds to one of the toxin factors or both.
- The complex thus formed passes into the cell by receptor-mediated endocytosis and into an acidified endosome after the conformational change on the toxin molecule.
- The edema toxin interacts with the host protein calmodulin and becomes an active adenylyl cyclase. The enzyme causes increased levels of cAMP and leads to hypovolemic shock.
- The edema toxin also has the ability to increase host susceptibility to infection by stimulating chemotaxis in human neutrophils.
- The lethal toxin then cleaves the members of the mitogen-activated protein kinase family interfering with certain signaling pathways and increasing the levels of shock-inducing cytokines like TNFα and IL-1β.
- During the early stages of infection as a result of the synergistic effect of both toxins, a reduction in the release of pro-inflammatory cytokines occurs, which significantly permits bacterial proliferation in the host.
- Lethal and edema toxins together can also induce vascular shock, but the nature of the shock might differ.
- Lethal toxin induces a cytokine-independent non-hemorrhagic vascular collapse with hypoxic necrosis, whereas edema toxin induces a generalized cAMP vascular dysfunction.
- Even though the primary targets of ET are the hepatocytes, epithelial cells are attacked during the cutaneous form of the disease, resulting in the characteristics phenotype of the lesions.
- As the infection continues, the bacteria make their way into the blood with the terminal levels of bacteria reaching, 107 to 109 cells/ml in susceptible hosts.
Figure: Pathophysiology of Anthrax. Image Source: NEJM.
Clinical Manifestation of Bacillus anthracis
The infection caused by B. anthracis is called anthrax which exits in three different forms depending on the route of entry of the bacteria. The most common form of anthrax infection is cutaneous anthrax which accounts for about more than 90% of all human cases. The other two forms of anthrax are gastrointestinal anthrax and pulmonary or inhalation anthrax. B. anthrax is also associated with meningitis.
1. Cutaneous Anthrax
- The period of incubation in cutaneous anthrax is about 2-3 days which in some cases might increase up to 2 weeks.
- Most of the exposure in cutaneous anthrax is either occupational or by the handling of infected animals or laboratory materials.
- The spores enter the body through breaks in the skin, and within 2-5 days, the skin bears the primary lesions.
- The primary lesions are characterized by painless lesions with pruritic papule, which converts into an ulcer in 24-36 hours with accompanying vesicles.
- The ulceration is followed by the drying up to form classic black eschar with eventual enlargement to cover the drying vesicle.
- Some of the lesions might be filled with pus in the case of secondary infection with pyogenic bacteria like Staphylococcus aureus.
- B. anthracis cells remain localized to the lesion in the case of uncomplicated anthrax, but adenitis of the surrounding lymph nodes might also occur.
- The eschar slowly begins to resolve after about two to six weeks of the appearance of the original lesion, irrespective of treatment.
- In the case of untreated anthrax, 20% or less of the patients might develop septicemia and die. With the use of appropriate antibiotics, however, the mortality rate is less than 1%.
2. Gastrointestinal Anthrax
- Gastrointestinal anthrax results from the entry of bacterial spores through the ingestion of undercooked meat from animals infected with B. anthracis.
- The incubation period is similar to a cutaneous infection, and a similar characteristic eschar is formed on the wall of the terminal ileum or caecum.
- Gastrointestinal anthrax can result in two clinical forms; abdominal and oro-oesophageal anthrax.
- In the case of abdominal anthrax, common symptoms like nausea, vomiting, anorexia, and fever are observed. As the disease progresses, severe abdominal pain with haematemesis, bloody diarrhea resulting in septicemia and death.
- In the case of oro-oesophageal anthrax, symptoms include sore throat, dysphagia, fever, and edema.
- Patients might accumulate massive ascites within 2-4 days after the onset of abdominal pain.
3. Pulmonary Anthrax
- Pulmonary anthrax accounts for about 2-5% of all the cases of anthrax. The pulmonary anthrax results from the inhalation of aerosolized spores.
- The infection begins with flu-like symptoms of mild fever, fatigue, and malaise that continue for 2-3 days after the initial exposure.
- The initial prodromal phase lasts for the first 48 hours and ends with the development of an acute infection with fever and cyanosis.
- The pulmonary system is considered a mode of entry rather than a site of primary pathology; thus, it is taken up by alveolar macrophages and transported to mediastinal lymph nodes rather than causing pneumonia.
- After 1-3 days of initial onset, the disease progresses to systemic dissemination with diaphoresis, fever, chills, and shock.
4. Meningitis
- Meningitis in anthrax occurs at the end stage of the other forms of anthrax. The symptoms appear rapidly with unconsciousness, but the progression is quite slow.
- The clinical symptoms include the appearance of blood in cerebrospinal fluid with eventual loss of shock or death.
Lab Diagnosis of Bacillus anthracis
The clinical diagnosis of B. anthracis infections is confirmed by the visualization and culture of B. anthracis from the clinical samples. The diagnosis of anthrax can be made through either conventional methods or molecular methods. The samples used for the identification of B. anthracis include swabs for collecting vesicular fluid in the case of cutaneous anthrax.
1. Cultural and Biochemical Identification
- This method is a conventional method for the identification of B. anthracis through growth on selective media, hemolysis test, capsule staining, motility testing, and susceptibility to penicillin.
- For the selective isolation of B. anthracis, PLET agar is used, which helps in the identification of B. anthrax through cultural characteristics.
- The presence of capsulated cells can also be used as a method of identification for B. anthracis by M’Fadyean staining with polychrome methylene blue.
- The conventional method of diagnosis of anthrax has some challenges due to the phenotypic and genetic similarity with other Bacillus species.
- The test of hemolysis can be performed to differentiate B. anthracis from B. cereus that is β-hemolytic.
2. Antigen-based Methods
- Antigen detection by immunoassay is an alternate approach to the diagnosis of B. anthracis.
- Common antigens used for these methods are glycoprotein BclA of the exosporium, extracellular antigen EA1 of the S-layer, the protective antigen of the anthrax toxin, and the poly-D-glutamine capsule.
- The selection of target antigen is dependent on the type of sample being tested as different antigens are expressed in the vegetative cells and spores.
- Common immunoassays used for B. anthracis identification are flow cytometry assays and luminescent adenylate cyclase assays.
3. Molecular methods
- Molecular methods like PCR involving the DNA amplification method facilitate the detection of B. anthracis without the culture of the bacteria, which makes them safer than the conventional methods.
- The commonly used genetic markers for the identification of B. anthracis are present on the virulence plasmids, pXO1 and pXO2.
- The genes used contain the coding component of anthrax toxins and the capsule. The detection of these genes also provides information on the virulence of the bacteria.
- However, there are some challenges with using these genes as the plasmids can be lost or transferred to other Bacillus species.
Treatment of Bacillus anthracis infections
- The treatment of anthrax is not very complicated as the bacteria is sensitive to many antibiotics like ciprofloxacin, erythromycin, penicillin, and vancomycin. It is resistant to cephalosporins, sulfonamides, and trimethoprim.
- Penicillin is the drug of choice as resistance against penicillin has not been found in naturally occurring strains.
- However, the rapid course of disease even with antibiotic therapy has resulted in high mortality rates for pulmonary anthrax.
- Therefore, antitoxin therapies have been studied and used to avoid the rapid progression of the disease and the removal of toxins.
- Currently, treatment with ciprofloxacin, amoxicillin, and doxycycline is recommended in the cases of mild cases of cutaneous anthrax.
Prevention of Bacillus anthracis infections
- Cases of human-to-human contagion have not been reported in the case of anthrax which suggests that the primary form of infection is the spore.
- Thus, the disease can be avoided by maintaining proper hygiene and protection during the handling of infected animals.
- Besides, active immunization is essential in anthrax pre-exposure prevention. The only toxin-based vaccine for B. anthrax approved by FDA is BioThrax.
- Even though it is supposed to be used pre-exposure, it is useful for post-exposure prophylaxis as well.
- Instruments and materials contaminated used on patients with anthrax should be autoclaved or incinerated as the usual practice.
B. anthracis as a bioterrorism agent
- The resistant spores of B. anthracis with the possibility of creating aerosol have the potential to use as a bio-terror weapon during war.
- The inhalation of spores is dangerous as the initial symptoms of the disease resemble that of flu, making the early diagnosis difficult.
- The fear of B. anthracis as a bioweapon has increased as it was supposed to be developed for use in World war I and II. In 2001, envelopes of B. anthracis were sent through the mail to different officials in the United States which are also considered an act of bioterrorism.






0 Comments