Millon’s Test Definition
Millon’s test is an analytical test used for the detection of the amino acids tyrosine, which is the only amino acid containing the phenol group. Millon’s test is a specific test for tyrosine, but it is not a specific test for protein as it also detects the phenolic group present in other compounds as well. Therefore, while performing Millon’s test, it is essential that other tests like the Biuret Test and Ninhydrin test also be performed. As many proteins consist of tyrosine, the test is useful in the detection of such proteins. The test was discovered by and named after the French Chemist Auguste Nicolas Eugene Millon.
Objectives of Millon’s Test
- To detect the presence of tyrosine-containing proteins in a given sample.
- To detect the presence of phenol-containing compounds.
- To differentiate tyrosine from other amino acids
Principle of Millon’s Test
Millon’s test is based on the principle of nitrification of the phenol group in tyrosine, which then forms complexes with heavy metals like mercury. The reagent used for the test is called Millon’s reagent, and it consists of mercuric nitrate and mercurous nitrate that is dissolved in concentrated nitric acid. In the test, the phenol group on the tyrosine molecule is nitrated by the nitric acid present in the reagent. The nitrated tyrosine then combines with the mercury ions in the solution to form a red-colored precipitate or solution. In some proteins containing tyrosine, the initial reaction between mercuric nitrate results in a white or yellow colored precipitate. After the addition of nitric acid and heating, however, the residue turns red in color. Both of these results are considered positive results and indicate the presence of tyrosine in the solution.
Requirements
Reagent
- Millon’s reagent: Millon’s reagent consists of mercuric nitrate and mercurous nitrate dissolved in nitric acid and distilled water.
Preparation of Millon’s reagent: Dissolve 160 grams of mercuric nitrate and 160 grams of mercurous nitrate in 400 ml concentrate nitric acid solution. The reagent is then made to 1000 ml by the addition of 600ml distilled water. The formula can be adjusted to suit the performance parameters.
- Sample (1% tyrosine)
Materials Required
- Test tubes
- Test tube stand
- Pipettes
- Water bath
Procedure of Millon’s Test
- About 2 ml of the sample solution or the 1% tyrosine solution is taken in a test tube.
- To this, about 2 ml of Millon’s reagent is added. The test tubes are then kept in the water bath for about 2 minutes if red colored precipitate is not observed immediately.
- The tubes are then observed for the formation of the colored precipitate.
Result and Interpretation of Millon’s Test
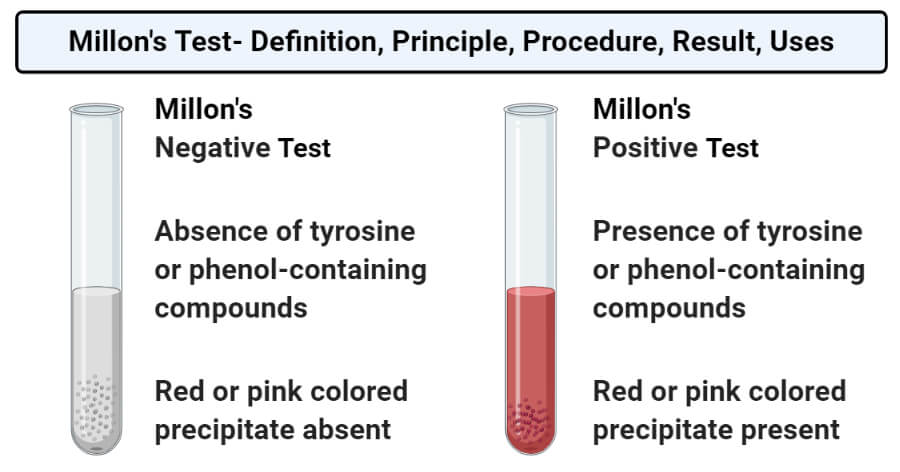
- Positive result: A positive result in the Millon’s test is demonstrated by the formation of a red or pink colored precipitate. This indicates the presence of tyrosine or tyrosine containing protein.
- Negative result: A negative result in the Millon’s test is demonstrated by the absence of colored precipitate in the test tube. This indicates the absence of tyrosine or tyrosine-containing protein.
Uses of Millon’s Test
- Millon’s test is used for the detection of tyrosine-containing proteins in a given sample.
- The test also helps in the differentiation of tyrosine from other amino acids.
- The test is useful in the detection of casein protein and the protein found in raw meat.
Limitations
- Compounds like salicylic acid and phenolic compounds give a positive result to this test; thus, any other phenol compounds that might be present in the test tube should be avoided. Tests like the Biuret test and Ninhydrin test should be performed for confirmation.
- The presence of chlorine in the solution might interfere with the reaction; thus, the test cannot be performed on a sample containing chlorides.
- The formation of a white or yellow precipitate might be observed immediately after the addition of Millon’s reagent due to the denaturation of proteins by mercuric ions.






0 Comments