Blood sample Types, Anticoagulants, Preservatives, Adverse effects of Additives
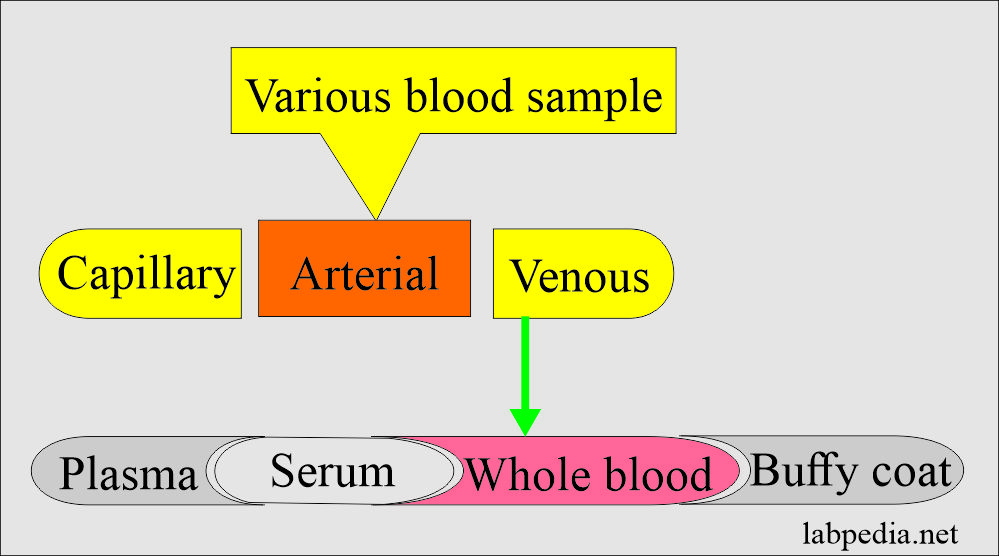
Types of the blood samples
Indications For The Whole Blood, Plasma, And Serum:
- A whole blood sample is used for blood gases and ammonia.
- It may be used for glucose, urea nitrogen, and lactate estimation.
- Serum and plasma are used for the majority of the chemical tests.
- The disadvantage of plasma is if you store the sample, then there are chances to form fibrin clots.
- These microclots may block the probe of the analyzer.
- Plasma is not a good sample for electrophoresis.
Type of patients for the blood samples:
- Pediatric patients: If this is the first time sample from the child, then gain his confidence.
- Blood for neonatal screening is collected to rule out hypothyroidism, phenylketonuria, galactosemia, and hemoglobinopathies.
- For phenylketonuria, take the blood at least 24 hours, and the infant has taken the feed.
- Adult patients: Be friendly and explain the procedure.
- Patients in the ICU are unconscious: No doubt, the patients are unconscious, but still, there may be a need to take the blood samples.
Types of blood samples and collection procedures:
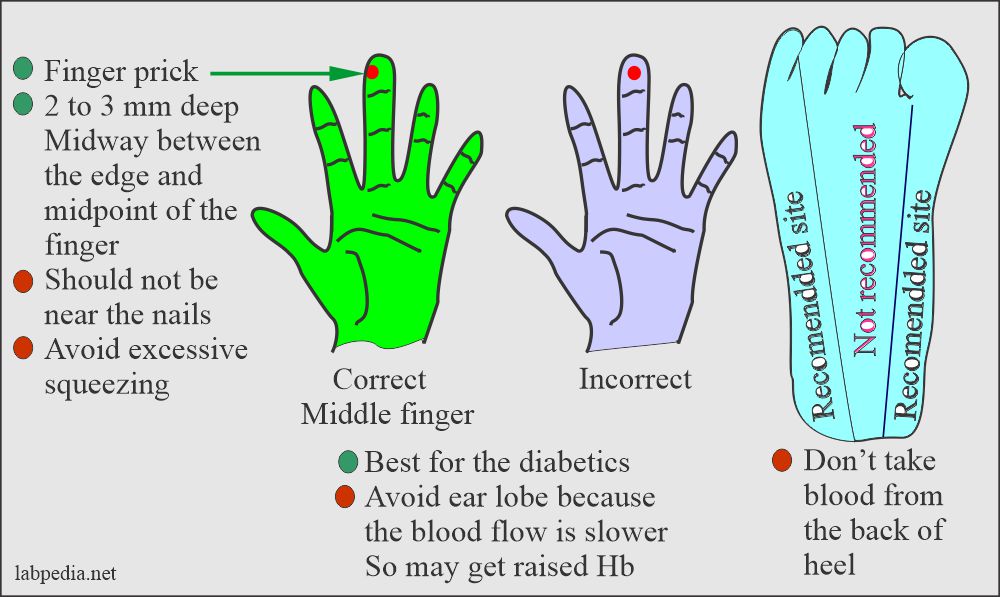
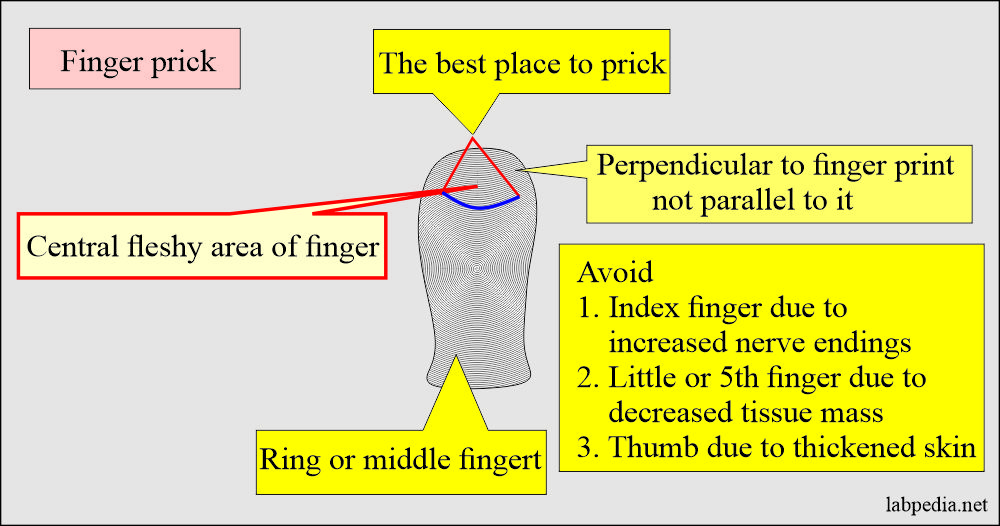
- Capillary blood (skin puncture).
- This is good for a small quantity of blood.
- Warm the finger from where taking the blood sample.
- In a newborn under 3 months, the heel is the best site to get a small blood quantity.
- The depth should not be >2.4 mm on the heel.
- Avoid the central portion and back of the heel.
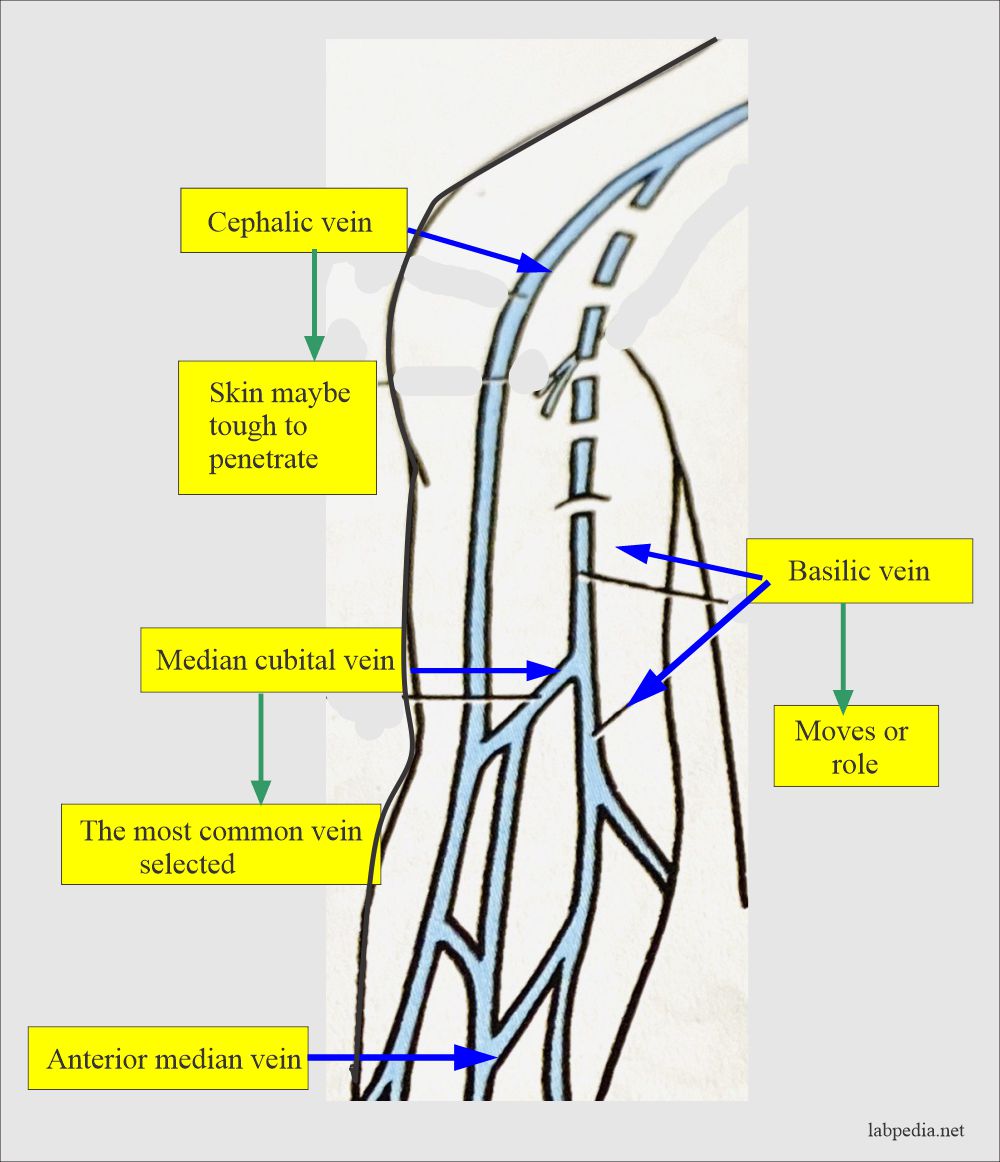
- Venous blood (venipuncture):
- For larger quantities, will take venous blood.
- The blood sample is taken from the forearm, wrist, or ankle veins.
- A forearm site is preferred. Blood is taken directly from the vein, called phlebotomy.
- The median cubital vein is usually preferred.
- Mostly venous blood is drawn in the fasting state.
- Blood collected after the meal is called a postprandial sample.
- There are biological variables in the blood collection like:
- Patient lying in bed or standing up.
- After the exercise.
- Diurnal variations.
- Recent food intake.
- Recent intake of Tea/coffee (caffeine), smoking (nicotine), alcohol ingestion, and drug administration.
- Can take a blood sample in vacutainers, syringes, and with the help of butterfly needles.
- The blood samples can also be taken for blood culture.
Difference between the values of venous blood and the capillary blood serum:
Capillary blood values < than venous blood No difference in capillary and venous blood values Capillary blood values > than venous blood. - Bilirubin = 5.0%
- Chloride = 1.8%
- Calcium = 4.6%
- Total proteins = 3.3%
- Sodium = 2.3%
- Phosphorus
- Urea
- Glucose = 1.4%
- Potassium = 0.9%
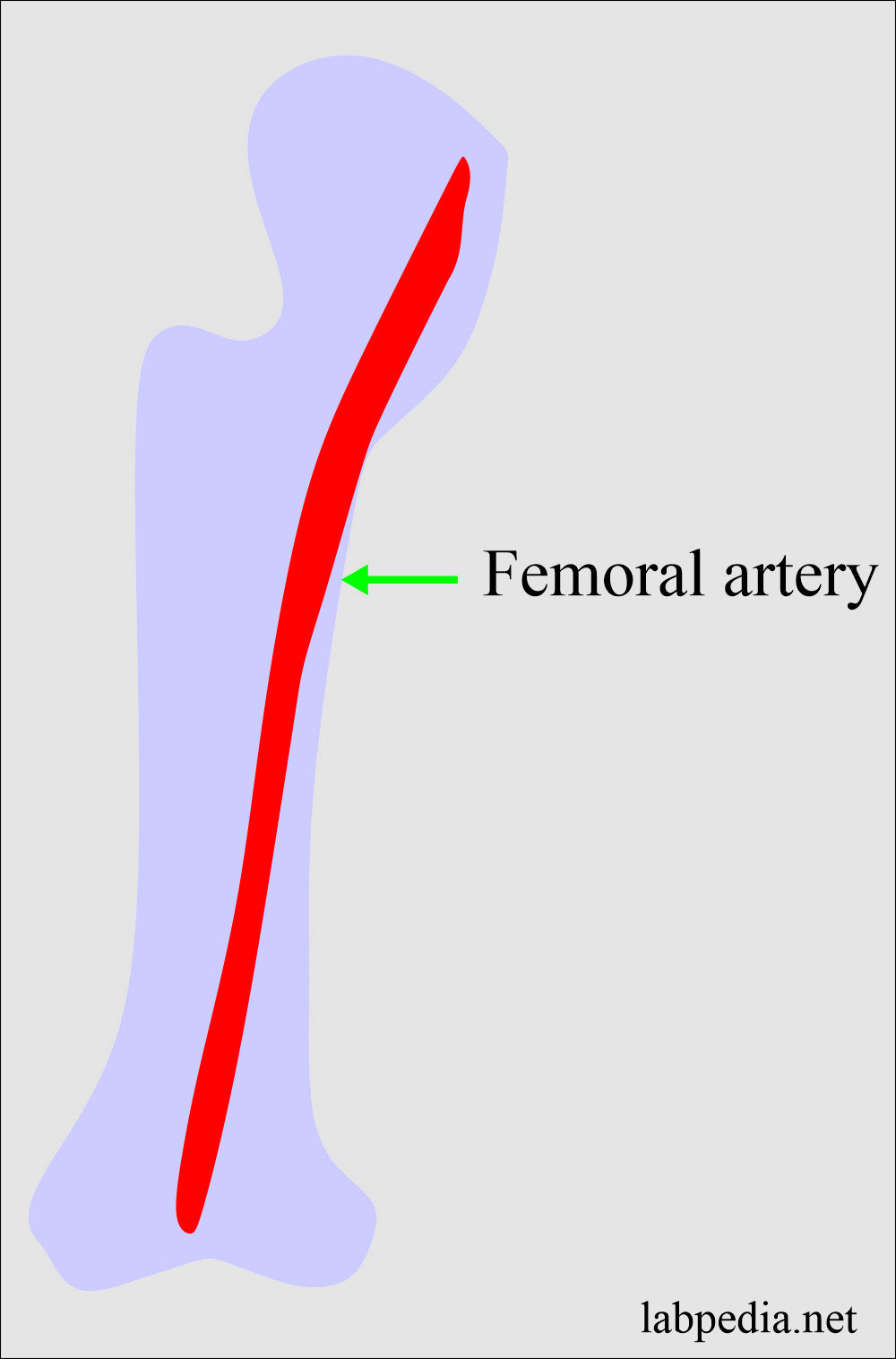
- Arteria blood is needed for the blood gases.
- Arterial blood is usually taken from the femoral artery.
- Blood for gases should be processed immediately without any delay.
Types of blood samples:
| Type of blood sample | Special features | Indications |
| Whole blood |
|
|
| Clotted blood |
|
|
| Plasma |
|
|
| Serum |
|
|
| Buffy coat |
|
|
Summary Of The Various Types Of Blood Samples:
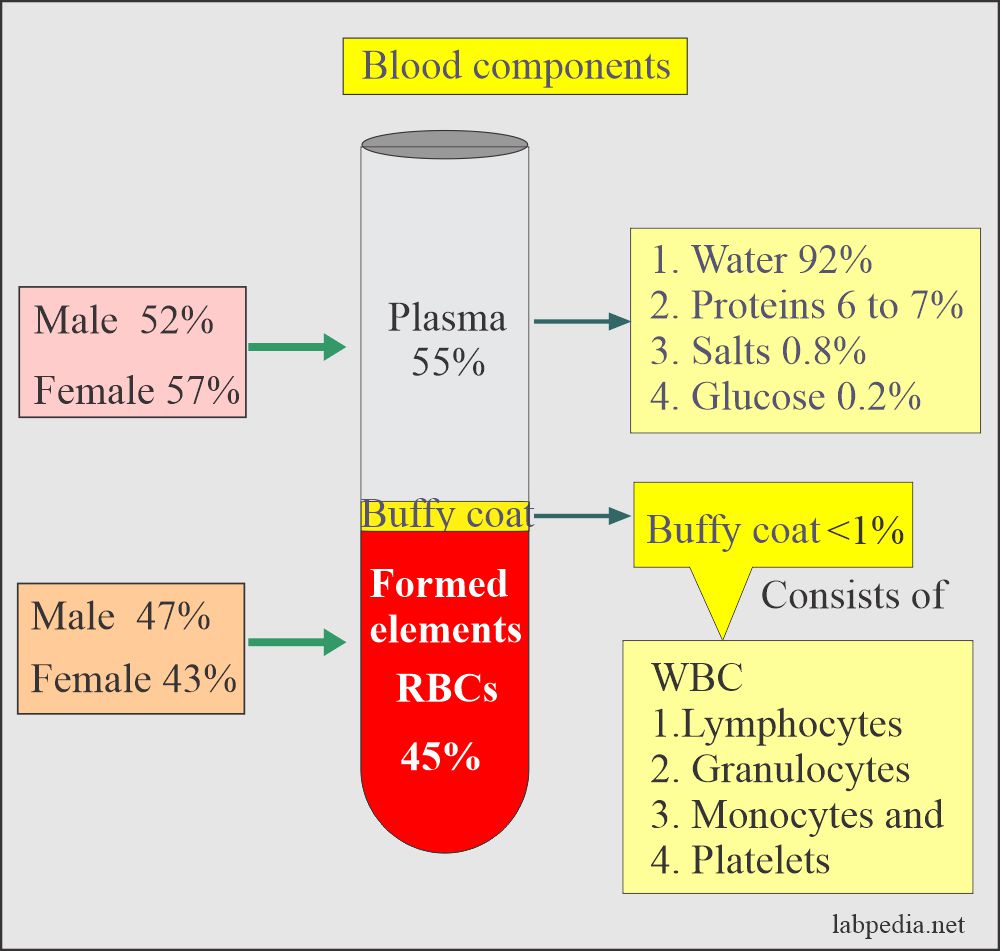
Whole Blood
- Obtain a blood sample in the test tube containing an anticoagulant.
- This sample will contain cells (white blood cells, platelets, RBCs, proteins) and plasma.
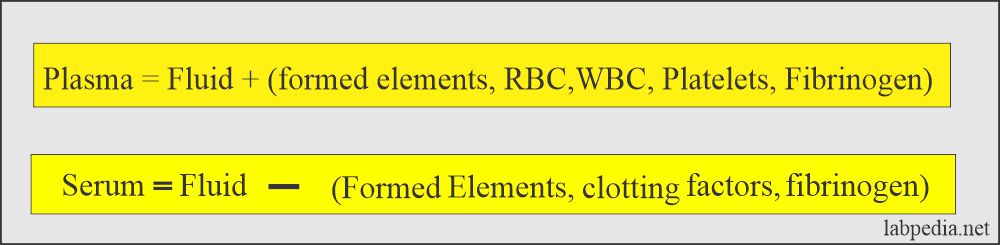
Plasma
- This is a pale yellow liquid that contains RBCs, white cells, and platelets.
- Plasma forms with the help of anticoagulants, which will prevent clotting.
- There is the presence of fibrinogen in the plasma.
Serum
- This is a clear fluid that is separated from the clotted blood. There are no RBCs, white cells, or platelets. There is no need for anticoagulants.
- Clotted blood is kept at 37 C for at least 20 minutes and then centrifuged.
- The upper portion is called serum.
- There is no fibrinogen.
Difference between the capillary and venous blood values:
Characteristic features Capillary blood/Venous blood No difference in values - Urea
- Phosphorus
Capillary value > than venous blood - Glucose = 1.4%
- Potassium = 0.9%
Capillary values < than venous blood - Bilirubin = 5%
- Total proteins = 3.3%
- Sodium = 2.3%
- Chloride = 1.8%
- Calcium = 4.6%
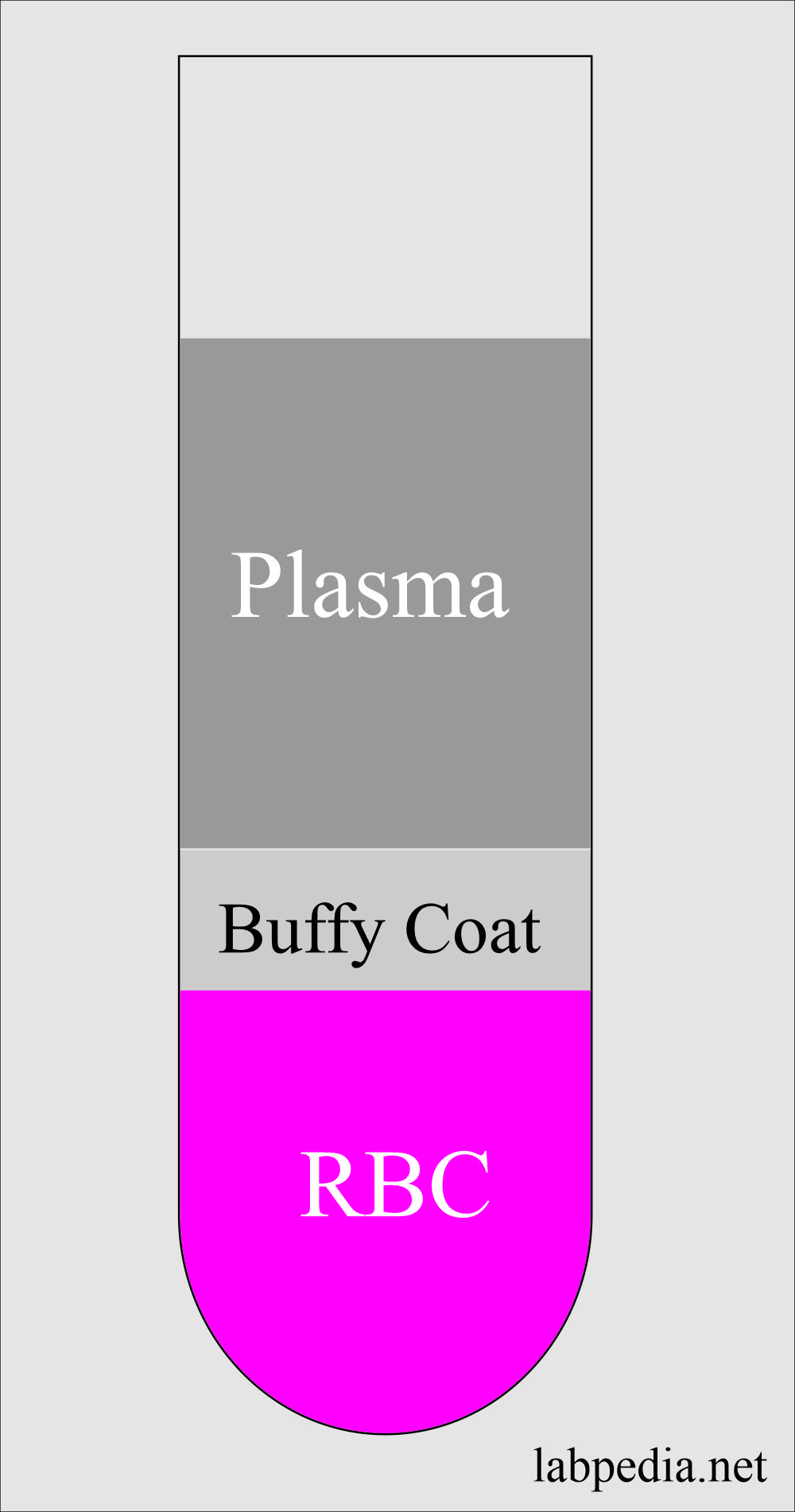
Buffy Coat
- This is the middle layer between the plasma and RBCs.
- This will contains white cells and platelets.
Table showing the difference between the contents of plasma and serum:
| Contents of plasma and serum | Plasma | Serum |
| Proteins | Contains all proteins (albumin, globulins, and fibrinogen) | Fluid remaining after coagulation |
| Fibrinogen | Contains fibrinogen | No fibrinogen |
| Water contents | 90% of water (92 to 95%) | 90% of water |
| Cellular elements | RBCs, WBCs, and platelets are suspended in plasma | No RBCs, No WBCs, No platelets |
| Electrolytes contents | Electrolytes same level | Electrolytes same level |
| Presence of prothrombin | No prothrombin | |
| Presence of antibodies | Antibodies are present | Antibodies are present |
| Presence of gases | Gases (CO2, O2, and N2) | |
| Presence of clotting factors | Fibrinogen |
|
| Presence of chemicals | Glucose, amino acids, cholesterol, and fats | Contains like plasma |
| Presence of hormones | Hormones | Contain rest of all products like plasma |
| Excretory products | Excretory products like urea, uric acid, creatinine, and bile | Excretory products present |
| Value of chemical substances | The same value of bilirubin, cholesterol, and creatinine | The same value of bilirubin, cholesterol, and creatinine |
The difference between plasma and serum:
Characteristics | Plasma | Serum |
| Fibrinogen | 0.2 to 0.4 G/dL | Nil |
| Formation site | Present in the body fluid | Prepared outside the body |
| Outside, the body contains | Always contains anticoagulant | Never anticoagulant added |
The following table elaborates on the composition between plasma and serum regarding the values of constituents of blood.
| Chemical substances | Plasma values more than serum | Plasma values less than serum. | No difference in the value in serum and plasma |
| Calcium | 0.9% | ||
| Chloride | 0.2% | ||
| Total protein | 4% | ||
| LDH | 2.7% | ||
| Albumin | 1.3% | ||
| SGOT | 0.9% | ||
| Alkaline phosphatase | 1.6% | ||
| glucose | 5.1% | ||
| Bicarbonate | 1.8% | ||
| Sodium | 0.1% | ||
| Phosphate | 7% | ||
| Potassium | 8.4% | ||
| Urea | 0.6% | ||
| Uric acid | 0.2% | ||
| Bilirubin | |||
| Creatinine | |||
| Cholesterol |
Purpose of anticoagulants
- To prepare the whole blood or the plasma, anticoagulants are needed.
- The anticoagulants are added to the container before collecting the blood sample.
- These are used to prepare the whole blood or plasma during the collection of blood samples.
- Definition of the blood:
- Blood is a combination of formed elements (RBCs, WBCs, Platelets) in a liquid portion called plasma.
- There is a difference in the plasma and the serum for estimating various substances in the blood.
Specimens to be rejected are:
- Sample with lipemia.
- Sample showing hemolysis.
- Specimens with contamination like not proper cleaning of the site.
- The sample quantity is not enough with the proper ratio for the tests.
In routine used anticoagulants are:
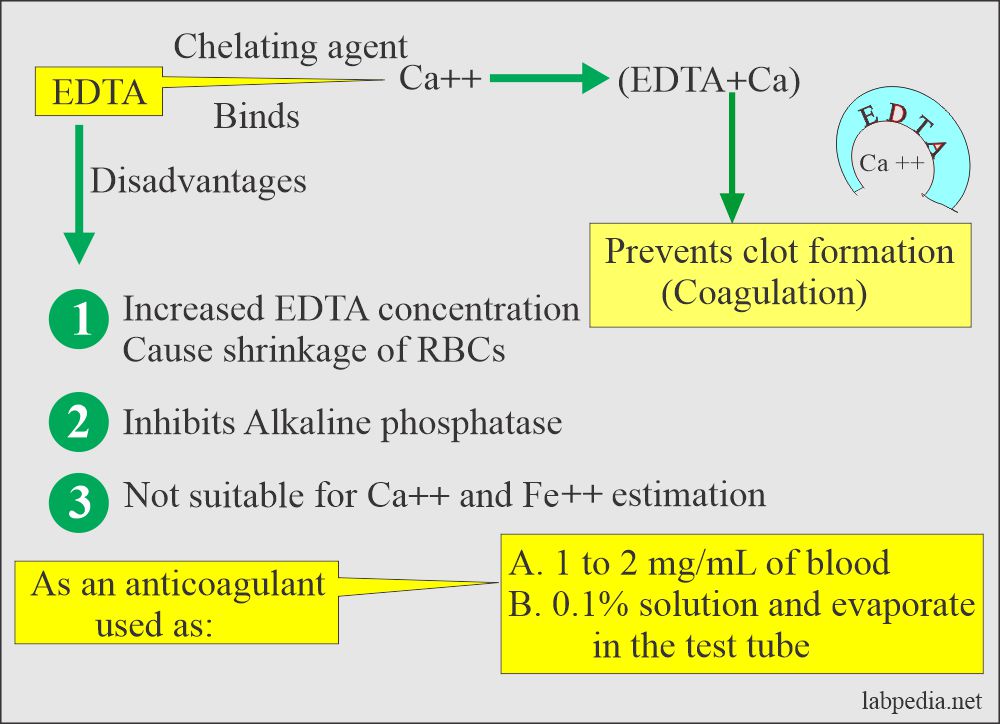
EDTA (Ethylenediaminetetraacetic acid)
- Indications:
- This is useful for the hematological examination.
- It is used for cell count, hematocrit, hemoglobin estimation, and the cell differential count.
- EDTA is used as a disodium or dipotassium salt.
- Mostly potassium EDTA is used as an anticoagulant, recommended for hematology studies. This is more soluble.
- Mechanism of action:
- This is a chelating agent that binds the calcium, which is needed for coagulation. Chelation prevents coagulation.
- It is effective at a final concentration of 1 to 2 mg / mL of blood.
- This can be used as a powder or make the solution and then add to vials. Let it dry.
- It is used as disodium, or dipotassium, or tripotassium salt.
- Solution:
- EDTA solution of 0.1% can be prepared and used. Let it evaporate at room temperature.
- Or 1.5 mg/mL.
- More than 2 mg/mL causes shrinkage of the cells.
- Advantages:
- EDTA preserves the morphology of the blood cell structure.
- This is the anticoagulant of choice for hematocrit, Hb, and differential count.
- This is the best anticoagulant for peripheral blood smears and studies.
- It has little effect on the various tests.
- They produce less shrinkage of RBCs.
- There is less increase in the cell volume after keeping the blood.
- Drawbacks:
- It inhibits alkaline phosphatase, creatine kinase, and leucine aminopeptidase activities.
- EDTA is not suitable for Calcium and iron estimation.
Heparin
Indications:
- This is used in the DVT (deep vein thrombosis)
- It is used in pulmonary embolism.
- This is also used in unstable angina.
- This is used as a prophylactic drug in venous thrombosis.
- If needed in pregnancy, this is the drug of choice because it can not cross the placenta.
- This is used in cardiopulmonary bypass surgery. This will maintain the patency of the blood vessels.
- It can be used in DIC if there are predominantly vasoocclusive manifestations.
- Low molecular weight heparin is given subcutaneously because this has a longer half-life than heparin.
- A prophylactically single dose is needed. Lastly, it is used as an anticoagulant and mostly used in hematology.
Properties of Heparin:
- This is an anticoagulant and causes the least interference with the test.
- This is theoretically the best anticoagulant because it is a normal blood component and does not introduce any foreign contaminants to the blood specimen.
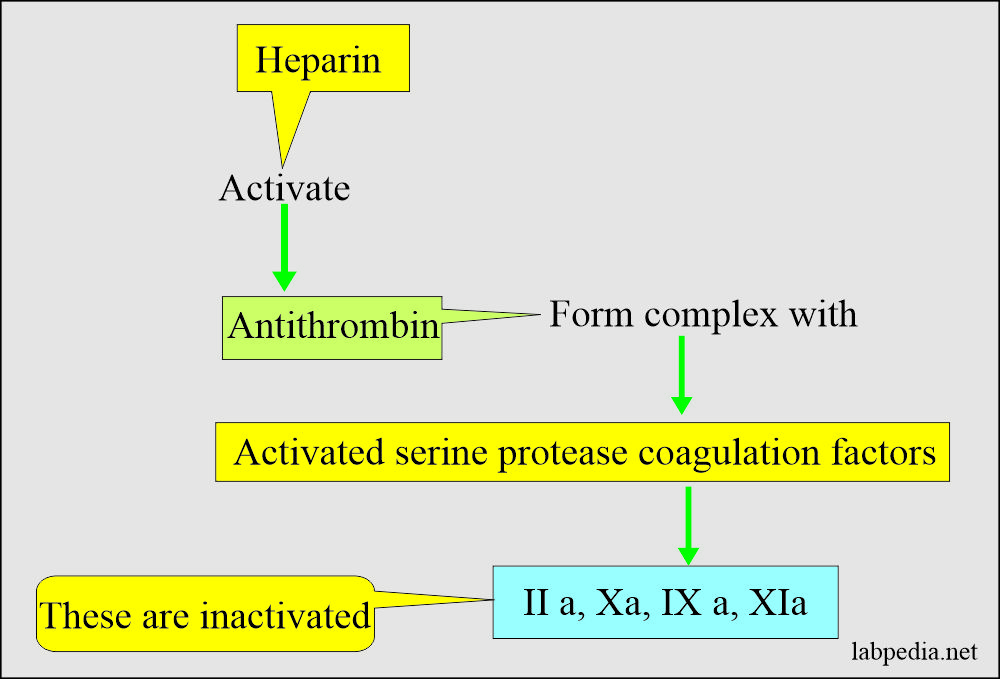
- This acidic mucopolysaccharide with a molecular weight of 15,000 to 18,000 is a blood coagulation inhibitor by potentiating the antithrombin activity.
- This is more costly than the others.
- It is present in powder form but is hygroscopic and dissolves rapidly.
- It is mucoitin poly sulfuric acid available as sodium, potassium, lithium, and ammonium salts.
- Mechanism of action of heparin:
- The GI tract does not absorb it, so given by injection in case of therapy.
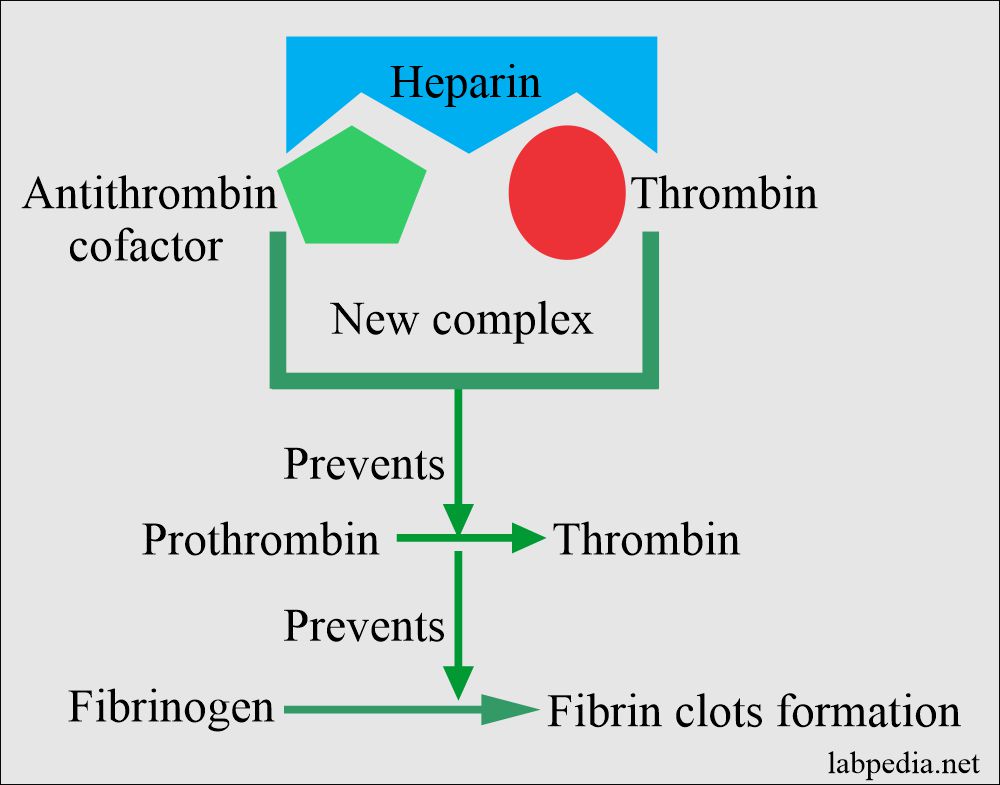
- Heparin accelerates antithrombin III action, which neutralizes thrombin, thus preventing the formation of fibrin from fibrinogen.
- It forms the complex of thrombin + antithrombin cofactor + heparin and prevents fibrin clot formation.
- It prevents the coagulation for 24 hours by neutralizing the thrombin, thus preventing fibrin clots’ formation from the fibrinogen.
- Solution preparation of the heparin:
- Heparin is added 0.2 mg / mL of blood in each test tube.
- Or 20 units of heparin for 1 mL of blood (in another reference, 15 U/mL).
- Or a drop of heparin is drawn into the syringe.
- Or simply coating the inside of the tubes or syringe is enough for the anticoagulant effect.
- After collecting blood, inverts the tubes 5 to 7 times for proper mixing of the blood.
- Advantage:
- This is the best anticoagulant to use dry when minimal hemolysis is desired, e.g., sodium and potassium estimation.
- This is the best anticoagulant used to estimate pH, blood gases, electrolytes, and ionized calcium.
- Drawback
- It is costly.
- It inhibits the acid phosphatase activity.
- It gives a blue background for Wright’s stain smears, so not good for peripheral blood smear interpretation.
- It also affects the binding of triiodothyronine and thyroxine to their carrier protein and produces a higher free concentration of these hormones.
- It interferes with the binding of calcium to EDTA.
- It is not used for coagulation and hematology studies.
- Ammonium heparin affects the RBCs volume.
Sodium Citrate
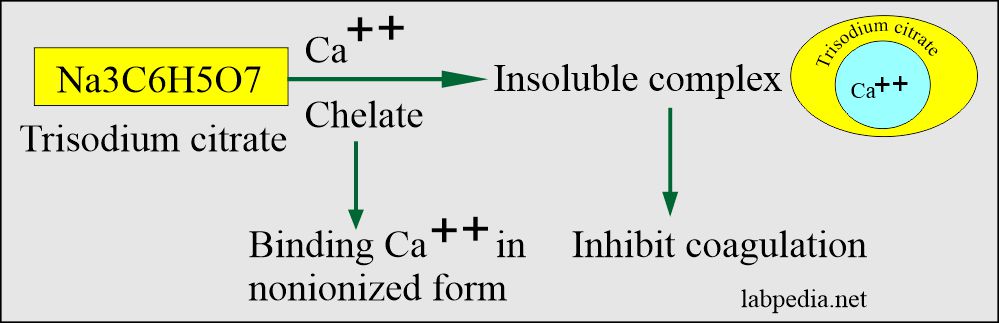
- Citrate is used as trisodium citrate salt.
- It is a white hygroscopic crystalline powder.
- Indications:
- Sodium citrate is widely used for coagulation studies.
- For PT and PTT.
- The sample can be used for ESR by the Westergren method.
- Mechanism of action:
- it is used in solution form.
- This will chelate calcium. Inactivates Ca++ ions.
- This will prevent the rapid deterioration of labile coagulation factors like factor V and factor VII.
- Solution preparation and uses:
- Trisodium citrate= 3.2 to 3.8 g/dL (3.2% solution).
- Mix well Trisodium citrate 3.8 grams in distle water.
- This can be used as 0.109 mg/mL.
- In blood, its ratio is 1:9, where 9 parts are blood, and 1 part is sodium citrate.
- PT and PTT= Blood: Sodium citrate = 9: 1 part (blood 9 parts: sodium citrate 1 part)
- ESR = Blood: Sodium citrate = 4:1 (1.6 mL of blood: o.4 mL Sodium citrate).
- Drawbacks
- This is used in liquid form (liquid anticoagulant).
- This is not a good anticoagulant for a complete blood examination.
- This is not good for the estimation of calcium.
- It inhibits aminotransferase and alkaline phosphatase.
- This will stimulate acid phosphatase when phenyl phosphate is used as the substrate.
- It has little value in clinical chemistry.
Potassium Oxalate
- Mechanism:
- This may be sodium, potassium, ammonium, or lithium oxalic acid salt used as an anticoagulant.
- This forms an insoluble complex with calcium ions (precipitate with calcium as a salt).
- This is the most popular oxalate salt used as an anticoagulant in powder form.
- Solution:
- Potassium oxalate at a concentration of 1 to 2 mg/mL of blood is used.
- Bulk solution: when you mix 30 grams/dL in distal water.
- Now add a few drops to the test tube side and dry it in the oven below 100 °C.
- The combination of ammonium/potassium oxalate does not lead to shrinkage of the RBCs.
- While other oxalates cause shrinkage.
- Drawbacks
- If the concentration is >3 mg/mL, then there are chances for hemolysis.
- There is a reduction of 10% hematocrit.
- Oxalates inhibit several enzymes like acid phosphatase, alkaline phosphatase, amylase, LDH.
- It may cause precipitation of calcium as oxalate salt.
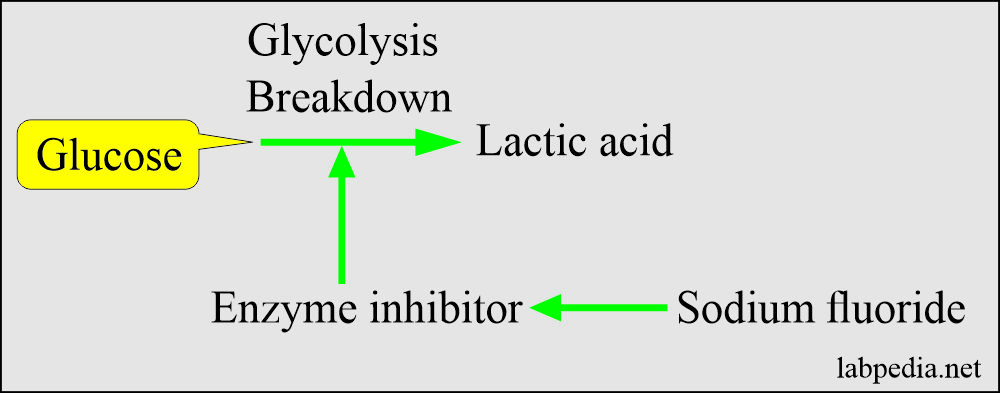
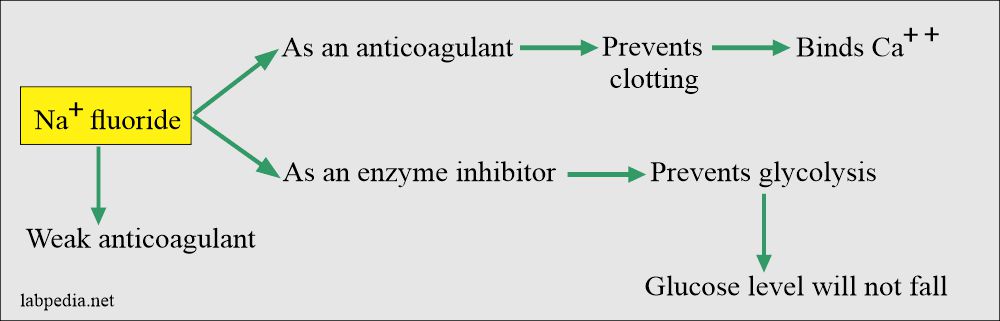
Sodium Fluoride
- This is a weak anticoagulant but used an antiglycolytic agent to preserve the glucose.
- This inhibits the system involved in glycolysis and preserves the glucose.
- This can be used as a dry additive.
- Mechanism of action: It acts in two ways:
- As an anticoagulant by binding the calcium.
- As an enzyme inhibitor that prevents the glycolytic enzyme from destroying the glucose.
- Sodium fluoride acts after the enolase, so it will not be effective in the first 1 to 2 hours. It prevents glycolysis after this period.
- Glucose can fall during this period, around 10 mg/dL.
- Transport on ice and rapid separation of the serum within 30 minutes can prevent glycolysis. There is no need for the addition of sodium fluoride.
- Not good for clinical chemistry tests.
- Solution:
- This is effective at a concentration of 2 mg/mL of blood along with another anticoagulant like potassium oxalate.
- When used alone, then more concentration than 2 mg/mL is needed.
- This can be used in combination with oxalate as a fluoride-oxalate mixture.
- Most specimens are preserved at 25 °C for 24 hours and at 4 °C for 48 hours.
- Sodium fluoride is poorly soluble, so mix blood thoroughly before effective anti-glycolysis occurs.
- This is mostly used for glucose estimation.
- The rate of decreases is faster in newborns because of the increased metabolic activity of the white cells.
- Drawback
- This is also an inhibitor of many enzymes.
- Also, effect urease for the estimation of urea.
Sodium Iodoacetate
- This is an effective antiglycolytic agent and substitute for sodium fluoride.
- This does not affect urease for glucose and blood urea levels instead of sodium fluoride and on a single sample.
- Solution use:
- It can be used at the concentration of 2 g/L and is an effective glycolytic agent.
- This may be substituted for sodium fluoride.
- This does not affect urease.
- Drawback:
- It inhibits creatine kinase but no effect on other chemistry tests.
Adverse effects of the additives:
- The additive may contain the substance to be tested like Na+oxalate for the estimation of Na+.
- The additive may remove the component to be tested like in oxalate, removes the calcium.
- The additive may affect enzymes like Na+flouride. This may destroy many enzymes.
- A small amount of the anticoagulant gives rise to microclots, and this will interfere with cell count.
- The additive may distort the cells like oxalate and change cell morphology like RBCs, which will become crenated. While WBCs show vacuoles. Lymphocytes and monocytes will have distorted shapes.
- If the excess quantity is used, that will dilute the substance to be tested.









0 Comments