Alkaline Phosphatase (ALP)
- It is done on the Serum of the patient.
- A fasting sample is a better choice. It is advised to fast 10 to 12 hours before taking the sample.
- This test can be done on the random sample as well.
- How to get good serum: Take 3 to 5 ml of blood in the disposable syringe or a vacutainer. Keep the syringe for 15 to 30 minutes at 37 C and then centrifuge for 2 to 4 minutes to get the clear serum.
- Keep the sample refrigerated as soon as you separate the serum.
- Because at room temperature level increases (up to 30% increase).
- The refrigerated sample also increases, but that is slow compared to the room temperature sample.
- Testing should be done on the same day.
- Serum at 0 to 4 °C is stable for 2 to 3 days, and at -25 °C is for one month.
- Perform the test as soon as possible because ALP activity increases 3 to 10% on standing at 25° C or 4 ° C for several hours.
Precautions
- Storage At room temperature increases the ALP activity.
- Avoid EDTA and oxalate anticoagulants or fluoride, which decreases Alkaline phosphatase activity.
- Reject sample in oxalate and citrate.
- If serum left at room temperature:
- Then there is a 1% increase in 6 hours.
- 3 to 6% in 1 to 4 days.
- Even though it may increase if refrigerated the serum, which is 2%/day, the increase is slow.
- This increase may be up to 30% if the serum is left at room temperature or kept in the fridge.
- Recent intake of food may increase the value.
- Values may be 25% higher after taking the high-fat meal.
- Drugs that increase the level:
- Drugs like allopurinol, antibiotics, colchicine, indomethacin, fluorides, isoniazid (INH), methotrexate, nicotinic acid, methyldopa, phenothiazine, vitamin D, and probenecid can increase the alkaline phosphatase level.
- It increases after a fatty meal.
- Drugs that decrease the level:
- Drugs like fluorides, arsenal, cyanides, nitrofurantoin, and zinc salts may decrease the alkaline phosphatase level.
- Hemolysis may cause a slight increase in the ALP. ALP is 6 times more in the RBC than the serum.
- Young children experiencing rapid growth, pregnant women, and post-menopausal females have a physiological high level of alkaline phosphatase.
- After I/V infusion of albumin, there may be a sometimes marked increase in alkaline phosphatase.
Indications
- Alkaline phosphatase is estimated to detect diseases of the liver and bone.
- Alkaline phosphatase is the best marker for obstructive jaundice.
- ALP distinguishes obstructive and hepatocellular jaundice.
- Alkaline phosphatase is the marker:
- For hepatic metastasis.
- In parathyroid diseases.
- Vitamin D deficiency.
- Acute pain in the abdomen.
Pathophysiology
Definition of ALP:
- The body contains many phosphatases. These are classified based on pH at which they will show maximum activity.
- ALP shows maximum activity between pH of 9 to 10.
- Intestinal mucosa shows the greatest activity, followed by kidney, bone, thyroid, and liver.
- Regan isoenzymes are observed in 5% of cases with carcinoma. It is identical to placental ALP isoenzyme.
The alkaline phosphatase functions:
- The exact metabolic function of ALP is still not known.
- The main function is to remove the phosphate group from the proteins and other molecules.
- These are necessary for the hydrolysis of organic phosphate and are important for digestion and mucosal absorption.
- ALP is associated with lipid transport in the intestine.
- The second role is in the osteoblastic tissues. The metabolic activity of the osteoblasts is associated with ALP activity.
- ALP is also associated with the calcification process of the bone.
- ALP is beneficial in distinguishing various bone diseases and hyperparathyroidism when combined with serum calcium and X-rays.
- Alkaline phosphatase is called Alkaline because its function is seen between a pH of 9 to 10 and best at a pH of 9.0.
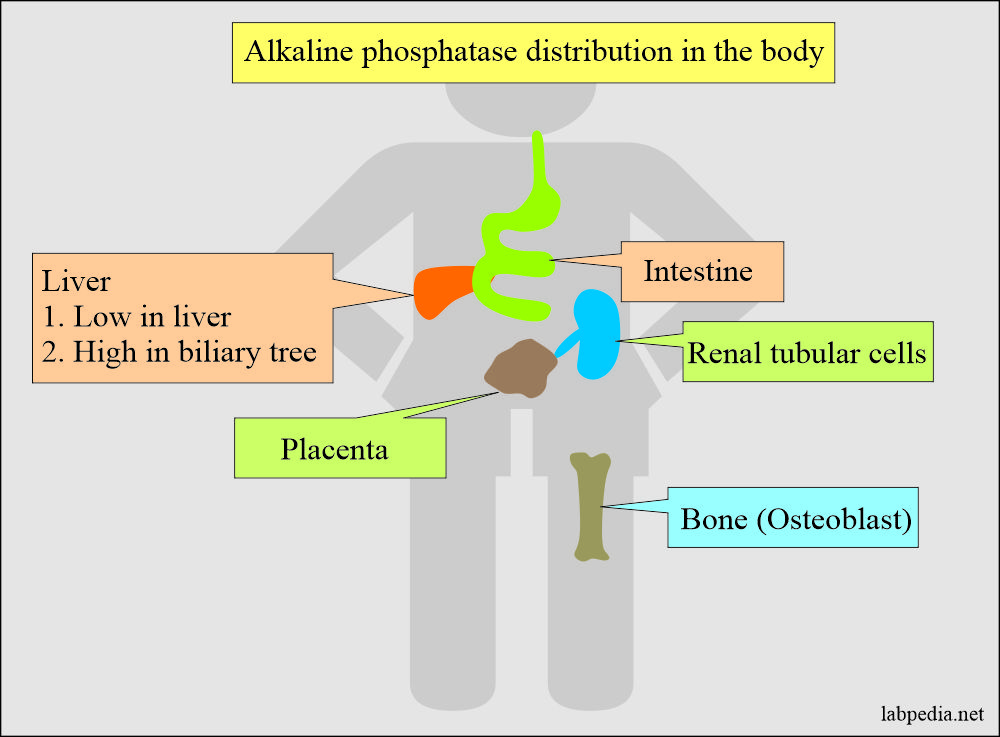
Distribution of the alkaline phosphatase:
- This is an enzyme present in the blood, and its subtypes are present in the liver, intestine, bones, and placenta.
- ALP is found in many tissues, at or in the cell membrane.
- ALP is a nonspecific enzyme capable of reacting with many different substrates.
- Liver and bone ALP are predominantly more in the serum.
- A small amount of the ALP from the intestinal epithelium is found in the sera of blood group B or O, who are secretors of blood group substances.
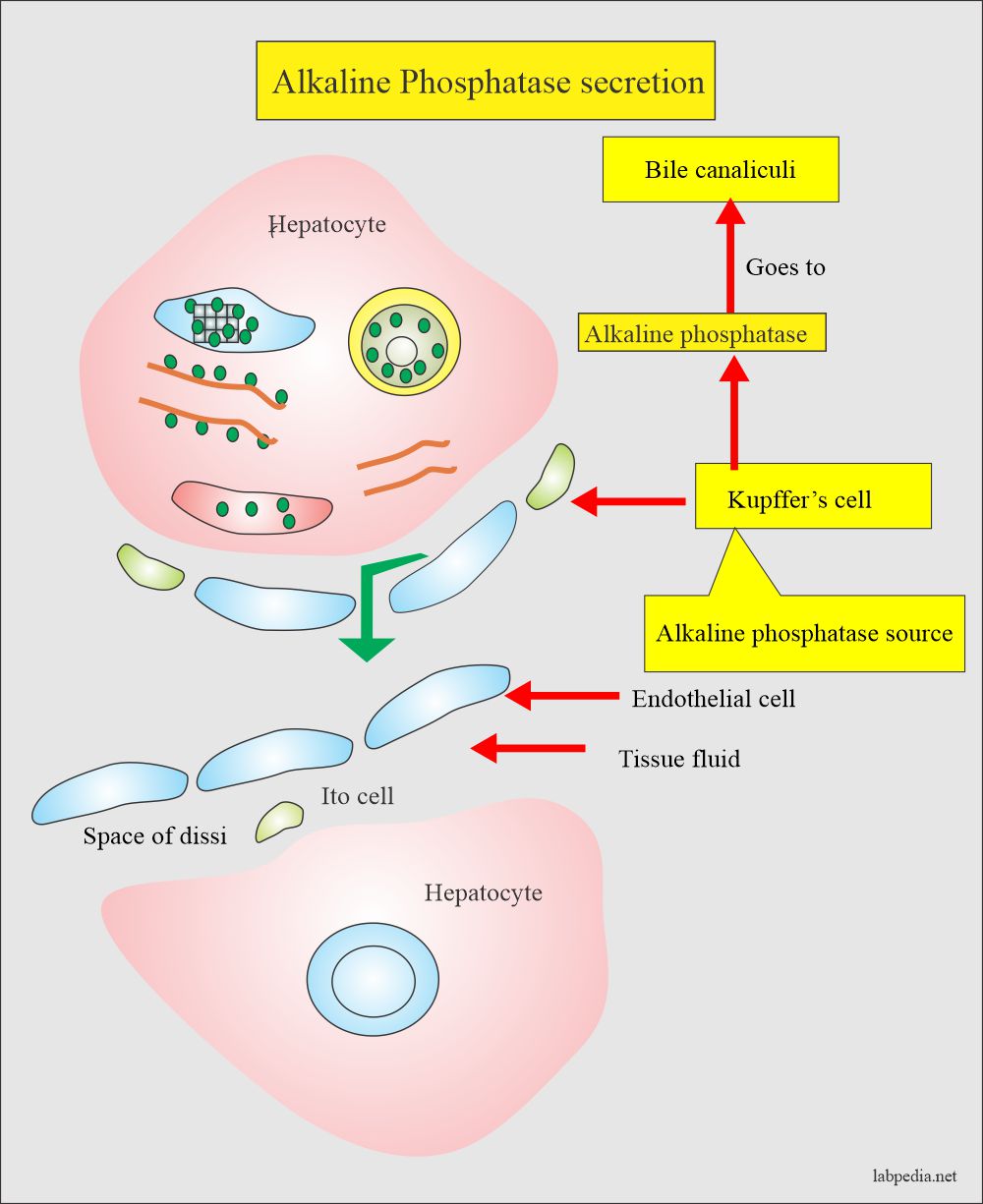
- The highest concentration is found in the liver. Within the liver, ALP is present in the Kupffer cells, and these cells line the biliary collecting system. From there, this enzyme is excreted in the bile. Its concentration in the liver is low as compare to the biliary tree.
- ALP is mainly from the liver, biliary tree, nearly up to 50% from the skeleton (osteoblastic cells), found in the intestinal epithelium, renal tubule cells, and lower concentration leucocytes and placenta.
- This ALP is age-dependent.
- Pregnancy can also lead to a raised level.
- Alkaline phosphatase in urine is from the renal tubule and not from the plasma.
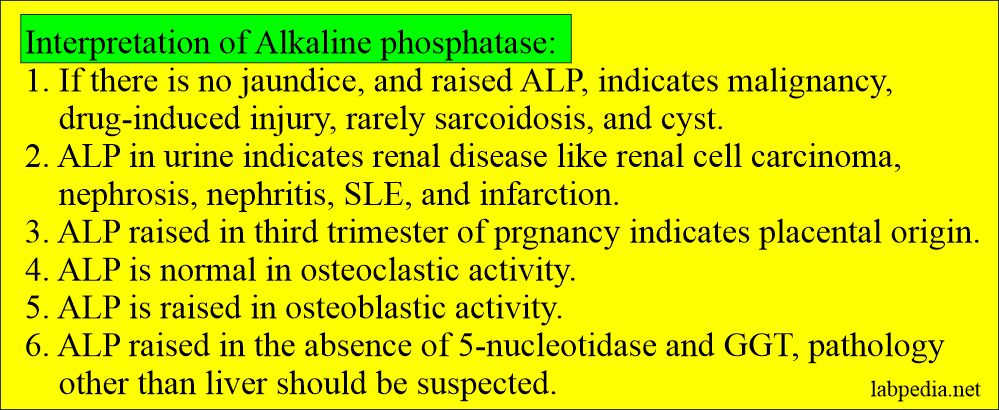
- This may be seen in renal lesions as a malignant tumor (carcinoma), nephrosis, nephritis, infarction, and systemic lupus erythematosus.
Effect of temperature on ALP:
- ALP (serum) is denatured at 56 °C and stable at lower temperatures.
- The liver isoenzyme is moderately heat stable at 55° C, but bone isoenzyme is heat-labile.
- Placental isoenzyme is stable at 65 °C but not others.
- Placenta ALP = Heat stable at 65 °C.
- Liver ALP = Moderately stable at 55 °C
- Bone ALP = Heat labile.
- ALP is distinguished by heating. The common method is heat fractionation, where serum is heated at 56 °C for 15 minutes and find the remaining ALP activity:
- Bone ALP activity will be only 10% to 20% of the original activity.
- The liver will retain ALP activity 30% to 50% of the original activity.
- Placenta ALP is heat stable and will contain virtually all of its activity.
- When serum is kept at room temperature will show increased activity.
- This varies from:
- 1% when kept for >6 hours.
- 3 to 6% >1 to 4 days.
- When kept in the fridge, it will still increase slowly to 2% per day.
- When frozen, then the activity decreases, which will recover slowly after thawing.
- This varies from:
- In lyophilized sera used for control as a reference also shows increased activity:
- 50 to 100% in 24 hours period.
- 10% when stored at 4 °C.
- 30% when stored at 20 °C.
- ALP enzymes require Magnesium for the reaction.
- Alkaline phosphatase is produced by the bone, bile ducts, intestine, and placenta.
- Post-puberty ALP is mainly from the liver.
- The conditions which will stimulate bone cells lead to an increase in the level of ALP.
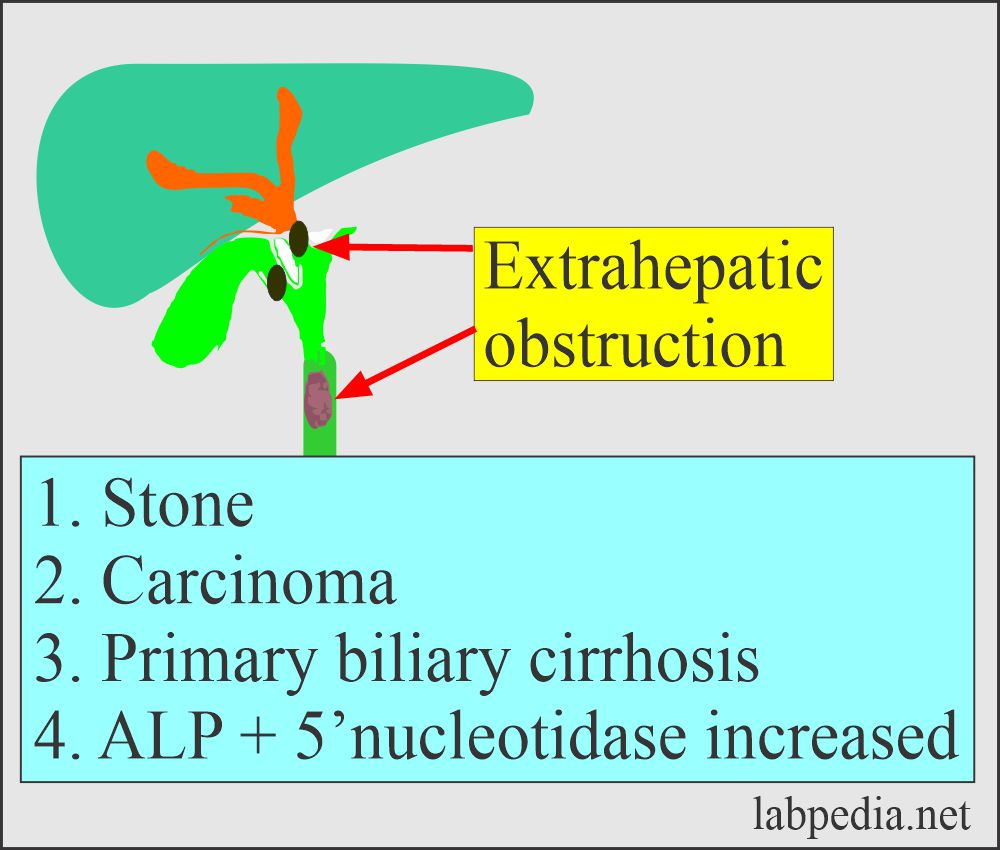
Effects of Obstruction on ALP:
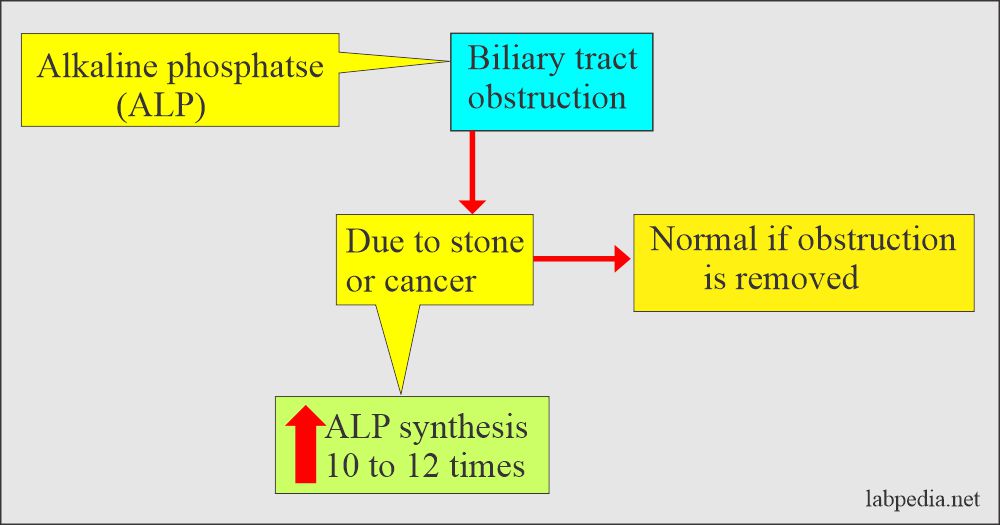
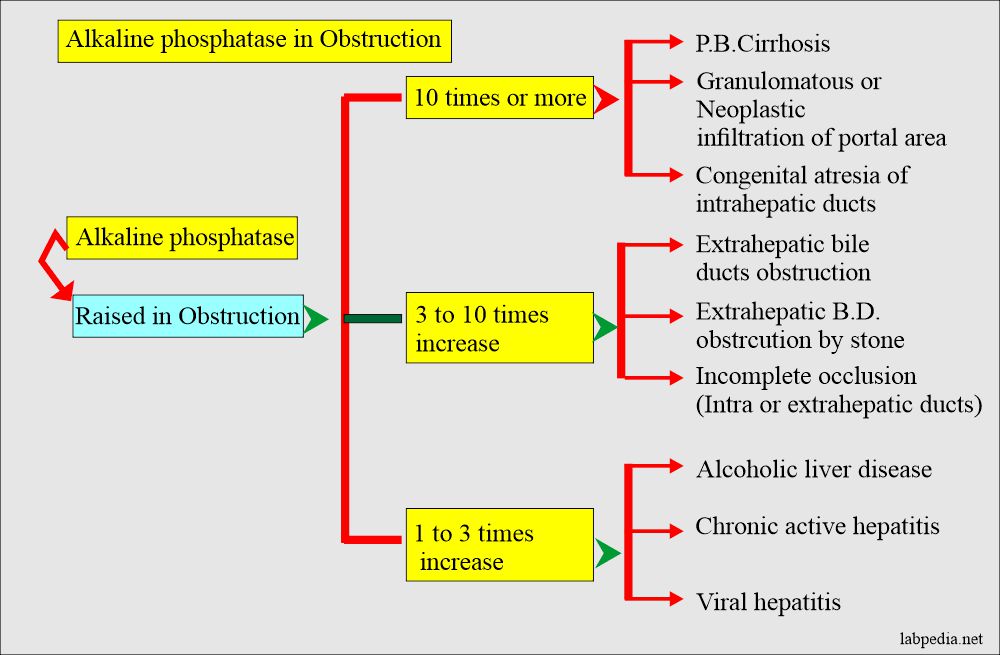
- The liver ALP is increased in biliary obstruction when its excretion is impaired. While in intrahepatic obstruction, there is less increase in ALP level than biliary obstruction, which may go up to 2.5 times.
- In extrahepatic obstruction, it may reach 10 to 12 times the upper limit and normal when surgically obstruction is removed.
- In the case of infectious hepatitis, there may be moderate elevation or even normal.
Effect of bone activity on ALP:
- The stimulus which increases bone activity causes an increase in the ALP level. So elevated levels indicate liver or bone diseases.
- Bone alkaline phosphatase is produced by the osteoblast of the bone and not from the osteoclasts.
- ALP level rises during active bone formation like in infants, children, and adolescents. So they have raised the level to 3 times the norm than the adults.
- Bone alkaline phosphatase indicates bone-forming activity.
The activator of the ALP enzyme are:
- Magnesium, Cobalt, and manganese.
- The exact ratio of Mg²/Zn² is important and to avoid the displacement of the Mg² to get optimal activity.
- Zinc is a constituent metal ion.
Inhibitors of the ALP enzyme are:
- Borate, phosphate, cyanide, and oxalate are inhibitors of the enzyme.
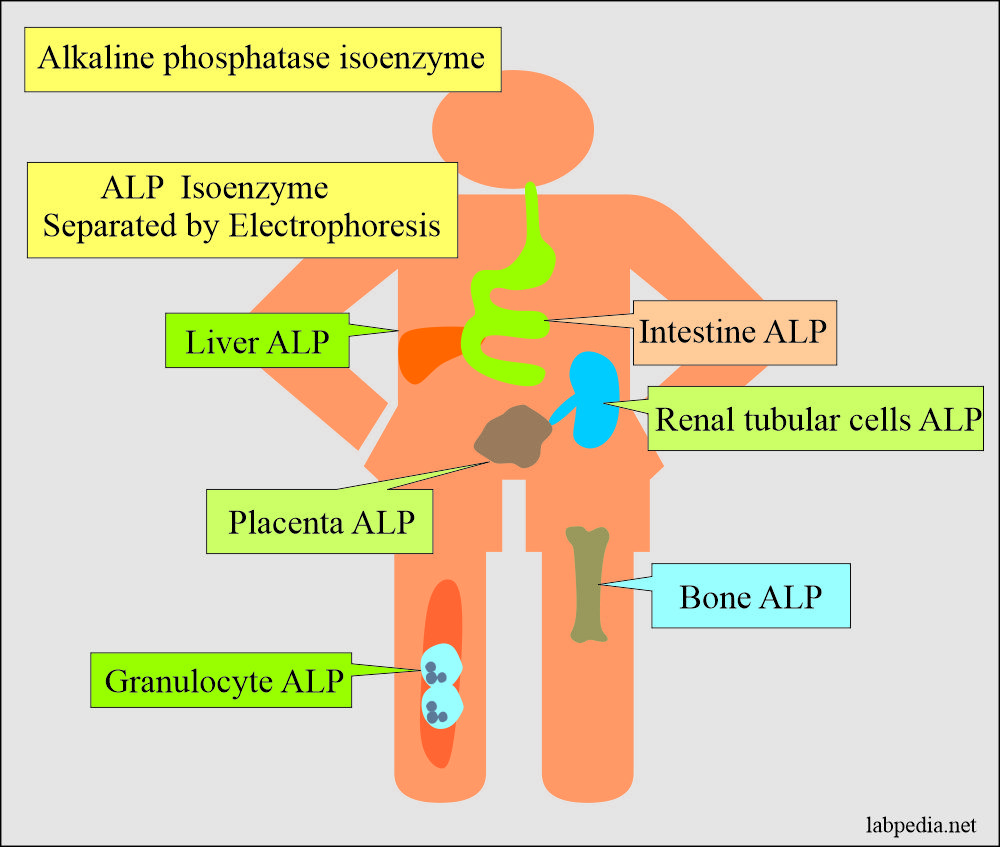
Alkaline Phosphatase Isoenzymes are:
The ALP is divided into isoenzymes based on:
- Acrylamide gel Electrophoresis.
- Characteristic inactivation by heating.
- Incubation and inhibition by the urea.
- Inhibition by the L-phenylalanine.
Isoenzyme of ALP Effect of heat/urea Effect of L-phenylalanine Electrophoresis (anodal mobility) Bone Positive (+++) Negative 2 (++) Liver and biliary Positive (+) Negative 1 Intestinal Positive (+) Positive (+++) 4 (++++) Placenta Negative Positive (+++) 3 (+++) Regan (Oncofetal placental ALP) Negative Positive (+++) 3 (+++)
Liver ALP isoenzyme (ALP1):
- 50% of the liver and biliary ALP is inactivated by the heat.
- This is derived from the epithelial cells of the biliary tract.
- The normal route of elimination is its excretion of bile into the intestine.
- This fraction moves fastest on electrophoresis, followed by bone, placenta, and an intestinal fraction.
- This is moderately heat-stable.
- This will be raised in liver diseases.
Bone ALP isoenzyme (ALP2):
- The heat inactivates 90% of the bone isoenzymes.
- This increases due to osteoblastic activity and is normally elevated in children and older people above 50 years of age.
- Children at growing age will have high-level ALP.
- Adults with healing fractures of the bone have raised levels of ALP.
- Paget’s disease leads to a very high level, 10 times or more upper limits of the normal.
- ALP is raised in metastasis to the bone, myeloma, and metabolic bone diseases like rickets, hyperparathyroidism, and osteomalacia.
- Deficient levels of this bone ALP are seen in the metabolic disorder of hypophosphatasia.
Intestinal ALP isoenzyme (ALP):
- It is ∼25% of the normal sera.
- This depends upon the blood group and secretor status of people.
- Blood group B or O and secretor are more likely to have this isoenzyme. In this group, it will increase ALP after 2 hours of the meal.
- This will leads to false positives in nonfasting individuals particularly Lewis positive type B and Osecretors.
- After the ingestion of the meals will increase the ALP by 30% for 2 to 12 hours.
- A fasting ALP level is indicated.
- This is inactivated by heat.
- This will be raised in inflammatory bowel diseases like ulcerative colitis and regional enteritis.
- This isoenzyme may lead to abnormal values in a non-fasting sample.
Placental ALP:
- Nothing of the placental ALP is inactivated by the heat.
- This is usually seen in the blood of a pregnant mother, which is placental in origin.
- Placental ALP appears 16th to 20th week of gestation. Then it keeps on increasing, two times the normal at the time of labor.
- It disappears 3 to 6 days after the delivery.
- This may increase the complications of the pregnancy like hypertension, preeclampsia, and threatened abortion.
- It is lower in diabetic than non-diabetic pregnancy.
- This oncofetal form of placental ALP is also referred to as Regan isoenzyme after the patient in whom it was first-time described.
Renal ALP:
- Renal tubular cells have ALP activity that is normally excreted in the urine.
- It does not reach the serum in pathological conditions.
- The sloughed renal cells may cause an artificial false-positive result on the new immunoassay screening procedure.
Granulocytes ALP:
- ALP in the granulocytes does not raise the serum ALP level.
- This is helpful as a marker for the granulocytic maturity in leucocytosis.
Alkaline phosphatase isoenzymes classification and differentiation:
| Basis for classification | Isoenzyme liver | Isoenzyme intestine | Isoenzyme bone | Isoenzyme placenta | Isoenzyme Renal |
| Amount of ALP | Present in the serum | Present in the serum in inflammatory GI tract diseases | Present in the serum | Trace amount found in serum | Excreted in urine |
| Heat stability | Stable at 56 °C for 30 minutes, more than bone | Disappear at 56 °C within 15 minutes | Disappear at 56 °C within 10 minutes | Stable at 65 °C for 30 minutes (Regan 15 to 15% cases of caners) | |
| Chemical inhibition | By urea but low by L-phenylalanine | Strong by L-phenylalanine | Strong by urea but low by L-phenylalanine | Resistance to urea but strong inhibition by L-phenylalanine | |
| Electrophoresis | Most anodic | Cathodic to the bone fraction | Intermediate | Migrate with the placenta or bone fraction | Renal isoenzyme more cathodic and slower |
| Gene arrangement | Same as the liver, the short arm of chromosome 1 | Product of unique gene long arm of chromosome 2 | Same as the liver, the short arm of chromosome 1 | Different Chromosome 2 | Same as the liver, the short arm of chromosome 1 |
Normal
- Value varies according to the kit and methodology used.
- As ALP exists in the various tissue, in the case of raised levels, isoenzymes may be advised.
- It is suggested that at least 1,5 times increase the need for further workup of the patient. If this value is found on 2 separate test results >6 months apart.
Source 6
| Adult Male | 25 to 100 U/L |
| Adult Female | 25 to 100 U/L |
| Children/adult | <2 years = 85 to 235 U/L |
| 2 to 8 years = 65 to 210 U/L | |
| 9 to 15 years = 60 to 300 U/L | |
| 16 to 21 years = 30 to 200 U/L |
Source 4
Male
- 1 to 12 years = <350 U/L
- 12 to 14 years = <500 U/L
- >20 years = 25 to 100 U/L
Female
- 1 to 12 years = <350 U/L
- >15 years = 25 to 100 U/L
ALP is slightly higher in the male than the female. It rises in females after middle age.
- There will be a biliary disease if = ALP raised + 5′-nucleotidase raised.
- If ALP raised + 5′-nucleotidase is normal, then think about other causes.
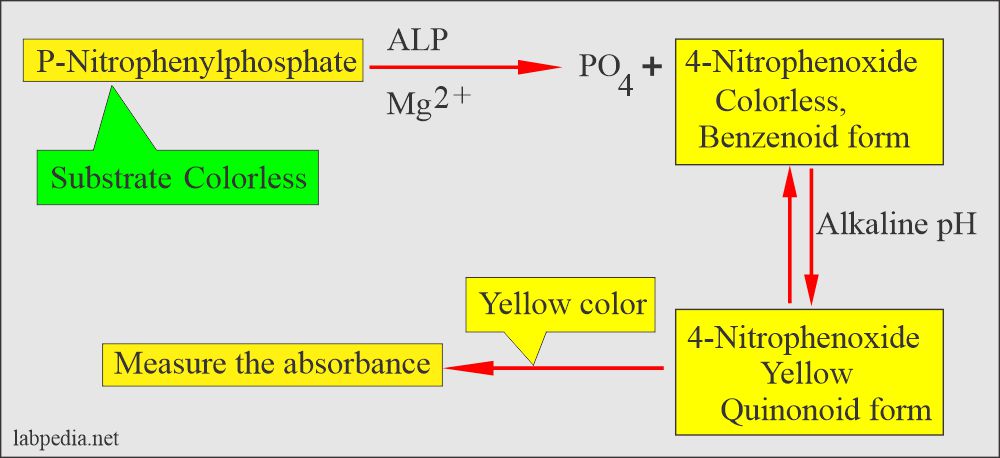
The principle of Alkaline phosphatase reaction:
Alkaline phosphatase is an enzyme of the hydrolase class that catalyzes orthophosphate cleavage from orthophosphoric monoesters under alkaline conditions.
There is colorless substrate p-Nitrophenyl phosphate (4-nitrophenyl phosphate) and by the action of ALP enzyme converted to p-Nitrophenol (4-nitrophenoxide, benzenoid form) is colorless, changed to 4-nitophenoxide, is the quinonoid form which is a yellow color in alkaline medium.
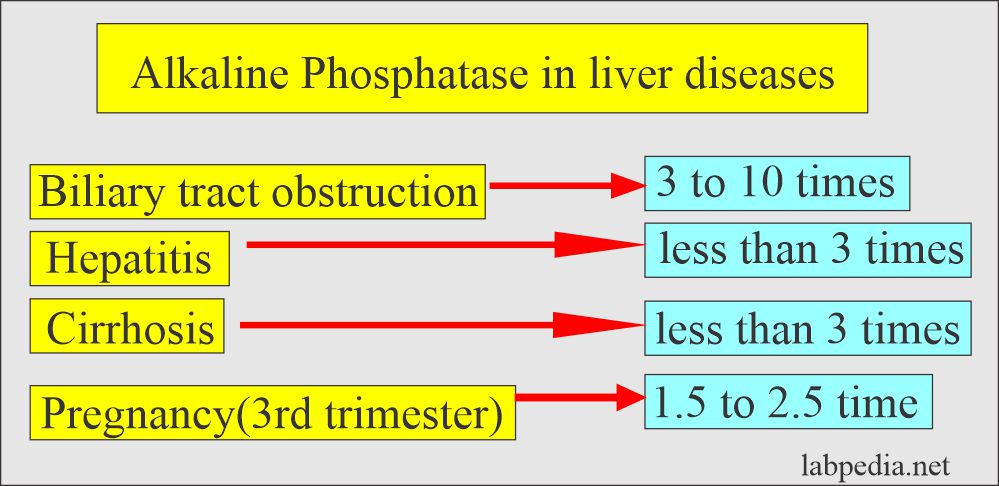
Alkaline phosphatase in liver diseases:
- In the absence of bone disease or pregnancy, markedly raised Alkaline phosphatase ( around 10 times ) is the best liver obstructive pathology marker like gallstones obstructing the biliary tract.
- Also, advise 5′-nucleotidase, and this will be raised in case of biliary disease. If it is normal then, search for other causes of raised ALP.
- 1 to 2 times raised level may be seen in various liver parenchymal diseases like Hepatitis and Cirrhosis.
- In extrahepatic obstruction due to stones and cancer, ALP increases 3 times the normal.
- In the case of complete obstruction, ALP may be raised 10 to 12 times.
- In the case of intrahepatic obstruction, ALP rises but less than extrahepatic obstruction.
- In the case of infectious diseases, ALP rises but <3 times.
- In pregnancy, the average increase in ALP is 1.5 times the normal limit between 16 to 20 weeks, and it persists till labor.
- After labor, ALP comes to normal within 3 to 6 days.
- This may be raised in pregnancy complications like hypertension, Eclampsia, pre-eclampsia, and threatened abortion.
- To confirm the hepatobiliary origin of ALP, advise γ-GT or 5-nucleotidase.
- ALP is a sensitive marker for hepatic metastasis. If there is hepatomegaly without jaundice indicates metastatic liver diseases.
Its level may be mildly raised in:
- metastatic diseases of the liver.
- Hepatocellular carcinoma.
- Biliary Cirrhosis.
- Intrahepatic and extrahepatic cholestasis.
- Gilbert’s syndrome.
- Chronic alcohol ingestion.
- Diabetes mellitus and diabetic hepatic lipidosis.
Alkaline phosphatase in bone diseases:
- It is increased in bone diseases like Paget’s disease, healing fractures, Rickets, pregnancy, and childhood.
- Metastatic bone tumors.
- Osteogenic sarcoma.
- Osteomalacia ( while in the osteoporosis ALP is normal ).
- There is a higher level of ALP in children, infants, and it is 3 times the adult level.
Alkaline phosphatase in bone diseases:
Disease | ALP Increased | Explanation |
| Paget’s disease | 10 to 25 times | Osteoblastic activity increase the ALP |
| Osteomalacia | Moderate increase | Level lowers with treatment |
| Ricket’s disease | 2 to 4 times | Level lowers with treatment |
| Osteoporosis | Normal level | |
| Physiologic bone growth | Increased level | |
| Healing fracture | mild, transient raised level | |
| Fanconi’s syndrome | Mild to moderate increase | |
| Primary and secondary hyperparathyroidism | mild to moderate increase | |
| Osteogenic sarcoma | Very high level | |
| Pregnancy third trimester | 2 to 3-time increase | This is the placental origin. |
| Children | 1.5 to 2.5-times increase | Growing age |
| Metastatic tumor-like Ca prostate | Raised level | |
| Solitary bone cyst | Normal |
Alkaline phosphatase increased level D/D due to obstruction of various degrees:
Other causes for raised alkaline phosphatase level:
- It is also raised in old age and pregnancy.
- Hodgkin’s disease.
- Sarcoidosis.
- Amyloidosis.
- Pulmonary and myocardial infarction.
- Hyperthyroidism (with a raised level of calcium).
- Chronic renal failure.
- Ulcerative colitis.
- ALP is increased during the last trimester of pregnancy and falls to normal within 3 to 6 days (postpartum).
- Hyperparathyroidism.
ALP Decreased level is seen in:
- Malnutrition.
- Hypothyroidism (Cretinism).
- Milk-alkali syndrome.
- Celiac sprue.
- Scurvy (vit. C deficiency).
- Gross anemia.
- Deposition of radioactive material in the bone.
- In hypophosphatemia.
- Pernicious anemia.
- Nutritional deficiency of zinc or magnesium.
- Theophylline therapy.
- Estrogen therapy in postmenopausal females.
- Wilson’s disease.
Alkaline phosphatase raised level in various condition:Level of raised ALP Causes of raised ALP >5 times of the normal (marked elevation) - Biliary cirrhosis
- Intrahepatic biliary duct obstruction
- Extrahepatic biliary duct obstruction
- Paget’s disease
- Hyperparathyroidism
- Osteogenic sarcoma
3 to 5 times of the normal (Moderate elevation) - Infectious mononucleosis
- Granulomatous infiltration of the liver
- Metastatic tumors in bone
- Rickets
- Osteomalacia
Up to 3 times the normal (Mild elevation) - Cirrhosis
- Viral hepatitis
- Pregnancy (placental ALP)
- Physiologic in children








0 Comments