Causes of Diabetes
- Absence of insulin: Pancreatic cells producing insulin are selectively destroyed by an autoimmune response, leading to Type 1 diabetes
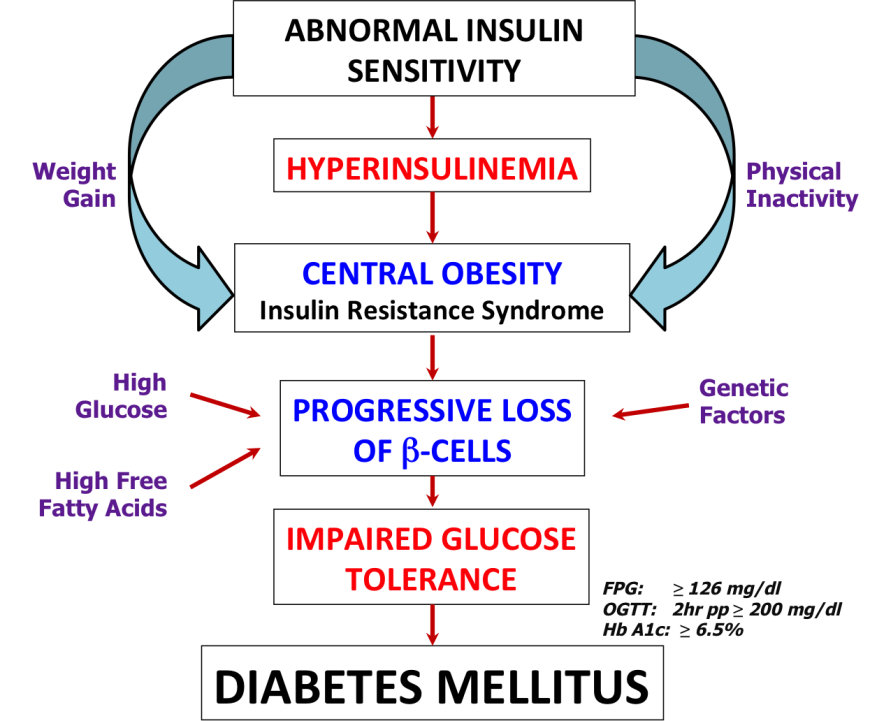
- Insulin Resistance: The individual may either have inadequate quantities of insulin in the body or problems with insulin function, both leading to Type 2 diabetes
- Inadequate insulin supply: Weight gain and obesity can lead to imbalance in the amount of insulin in relation to sensitivity of the cells to insulin
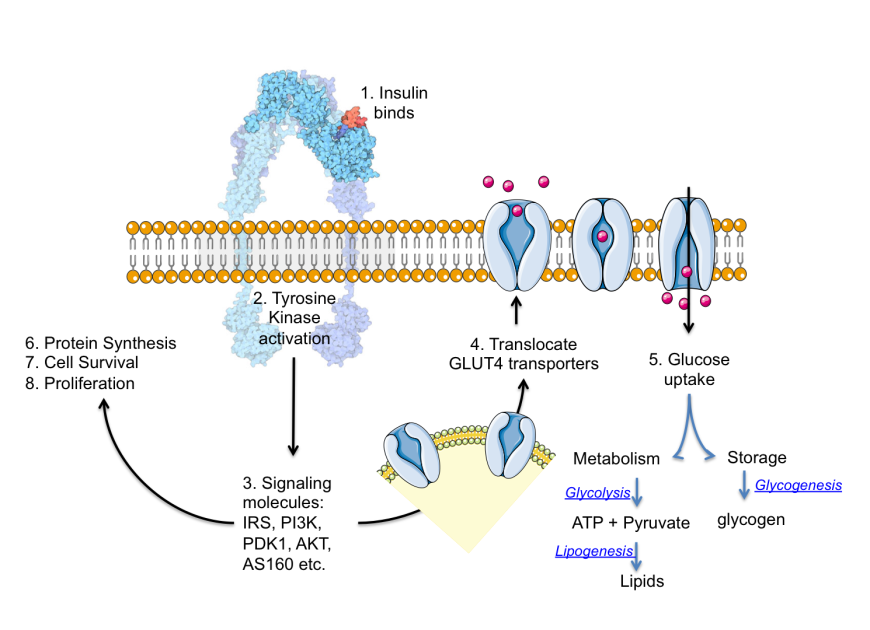
- Improper insulin function: Insulin binds to its receptor and initiates a cascade of signals in the cell, ultimately leading to the uptake of glucose. Missing or defective proteins in the signaling cascade (due to genetic defects or mutations) and/or changes in metabolites or signaling molecules (such as high levels of free fatty acids) may disturb the metabolic balance, leading to diabetes.
Types of Diabetes
| Type | Causes/Characteristics | Clinical Profile/Comments |
| Type 1 Diabetes | Autoimmune; also knows as juvenile or insulin-dependent diabetes | Insulin is either not produced or produced in insufficient amounts due to β-cell destruction; requires supplemental insulin |
| Type 2 Diabetes | Polygenic; influenced by environment and lifestyle; also known as adult-onset or insulin-independent diabetes | Decreased responsiveness to insulin; increased incidence in individuals with obesity and family history of diabetes due to dual defects - insulin resistance and impaired insulin secretion capacity |
| Gestational Diabetes | Subset of Type 2 Diabetes; diagnosed as glucose intolerance during pregnancy | Insulin resistance; condition may persist after pregnancy |
| Prediabetes | May be genetic or environmental | May have arisen due to obesity and family history; usually can be mitigated by lifestyle changes |
| Maturity-onset diabetes of the young (MODY) | Monogenic; inherited in an autosomal dominant pattern; early age onset of hyperglycemia (generally before age of 25) | Impaired insulin secretion with minimal or no defects in insulin action |
| Secondary Diabetes | Induced by side effects of medications, infections, pancreas dysfunction (e.g. due to use of steroids, alcoholism, pancreatitis, etc.), or rare autoimmune disorders | Loss of pancreatic function, insulin resistance or impaired insulin secretion, depending on the specific cause(s) |
Symptoms
- Polyuria or the need to urinate frequently helps the body remove excess glucose that is filtered from the blood by the kidneys
- Polydipsia or increased thirst and fluid intake compensates for the loss of fluids resulting from increased urination
- Polyphagia or increased appetite compensates for the loss of glucose and fluids from the body, caused by excessive urination
- Extreme fatigue
- Blurry vision
- Slow healing of cuts/bruises
- Weight loss
- Tingling, pain, or numbness in the hands/feet
- Fainting and/or dizziness (possibly due to sudden drops in blood glucose levels due to poor management or increased medication)
- Foot ulcers (resulting from inability to attend to foot injuries due to loss of sensation in the extremities. These conditions may lead to gangrene and amputation)
- Peripheral neuropathy (loss of sensation, autonomic dysfunction)
- Retinopathy (blindness, blurred vision, or eye problems)
- Nephropathy (kidney damage)
- Damage to nerves (usually affects the extremities first) and bloods vessels, exposing diabetics to risk of cardiovascular complications (e.g., high blood pressure, heart disease, heart attack, stroke)
- Increased susceptibility to infections (e.g., urinary tract infections, sepsis, gangrene), since high glycemic environment provides a favorable medium for microbes
Diagnosis
- Plasma glucose levels: The American Diabetes Association reports that the fasting plasma glucose (FPG) for normal individuals should be <100 126="" 140="" 2="" 8="" a="" after="" approximately="" as="" at="" be="" caloric="" defined="" diabetes="" diagnosis="" dl.="" dl="" fasting="" for="" glucose="" greater="" here="" hours="" implying="" intake="" is="" least="" less="" levels="" meal="" mg="" no="" occasions.="" of="" on="" overnight="" plasma="" requires="" should="" span="" than="" the="" two="" usually="">
Test Administration Risks Blood is drawn from the vein following overnight fasting (for fasting blood glucose levels) or at any random moment during the day (to measure random glucose levels). There may be some bruising, infection, and soreness at the site of puncture for drawing blood. The subject may also feel some dizziness.
- Oral Glucose Tolerance Test (OGTT) is an elaboration of the blood glucose test that reveals how the body metabolizes glucose ~2 hours after ingesting glucose. For pregnant mothers with high risk of diabetes, a glucose-screening test (OGTT) is usually performed between 24-28 weeks of pregnancy (or earlier) to identify gestational diabetes and manage the blood glucose levels for the health of mother and baby.
Test Administration Risks After blood is drawn, the subject is asked to drink a liquid, which contains 50, 75, or 100 grams of glucose. Normally the absorption of glucose in the body occurs rapidly, and blood glucose levels rise within 30 to 60 minutes of fluid intake. Blood work is done an hour after drinking the solution. If the blood test shows abnormal blood glucose levels, then blood work must be repeated after three hours. The blood test may cause side effects such as moderate pain, nausea, and faintness. Drinking the glucose solution may cause symptoms like shortness of breath, nausea, and vomiting, particularly in pregnant women with a predisposition to gestational diabetes mellitus.
- Hemoglobin A1c (or Hb A1c or A1c)refers to the percentage of glycated hemoglobin present in the blood. Prolonged exposure to high blood glucose levels leads to glucose attaching itself to proteins including hemoglobin. Since this happens over a period of time and is irreversible - the HbA1c test is not affected by random fluctuations due to temporary alteration in diet, lifestyle, stress or illness of the subject. Maintaining an HbA1c below 5.7% is indicative of normal health. An HbA1c level of 5.7-6.4% signals prediabetes, and any values higher than 6.4% indicates a diabetic condition requiring treatment.
Test Administration Risks A sample of blood is taken either from a finger stick or by drawing a small vial of blood from a vein (venipuncture) and is tested. Obtaining a blood sample for the test may lead to light-headedness or fainting of the subject. It may also lead to infection, excessive bleeding, or hematoma (localized abnormal accumulation of blood under the skin) at the site of puncture for drawing blood.

Complications
- Ketoacidosis: Despite high blood glucose levels, the body cells (muscle and lipid cells) may be starved for glucose due to absence or improper function of insulin. Cells may also starve for glucose if the amount of food intake is low (such as during illness) or if the dosage of insulin is too high. Under these conditions cells start using fats as a source of energy. Liver cells produce ketone bodies from fatty acids. When the glucose levels are low, brain cells can use ketone bodies, but not free fatty acids, for energy. High concentrations of ketones can make the urine acidic and cause fruity-smelling breath. If not managed, this condition can progress to coma (prolonged unconsciousness) and even death.
- Hyperosmolar Hyperglycemic Nonketotic Syndrome (HHNS): High blood glucose level triggers increased urination. If liquids are not replaced, the individual can become severely dehydrated. High blood glucose levels can lead to altered mental states, confusion, seizures, coma, and even death.
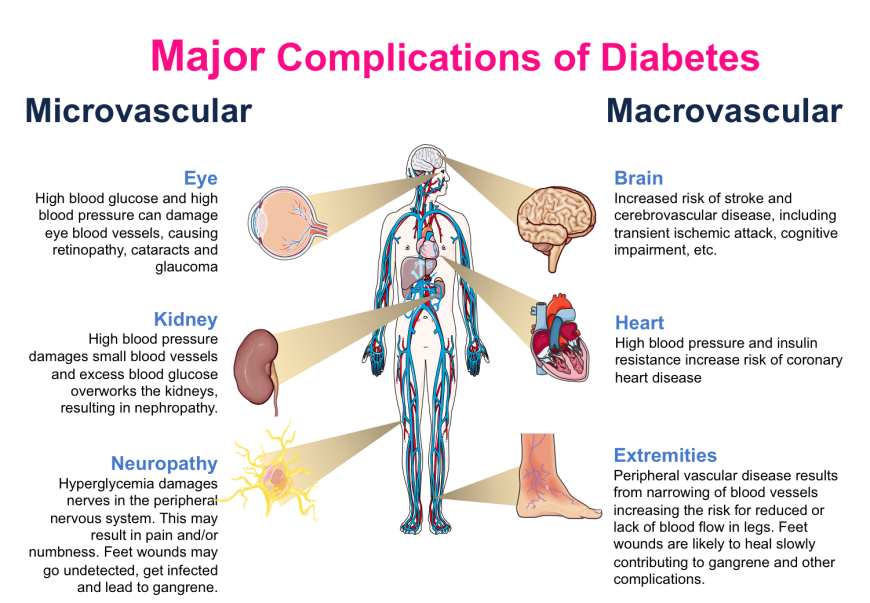
- Microvascular complications affect the smaller blood vessels, such as in the eyes (leading to retinopathy), kidneys (leading to nephropathy), and neurons (leading to neuropathy). Individuals with poorly managed blood glucose levels may suffer from one or more of these complications in advanced stages of the disease. Thus, besides monitoring the health of eyes and kidneys, diabetics also require foot care. Interestingly, several large population studies have shown that aggressive management of blood glucose levels (i.e., keeping blood glucose levels within a narrow range) can avoid, or at least delay, the onset of these complications (Nathan et al., 2014). Regular monitoring and management of blood glucose levels is of critical importance in maintaining metabolic balance and avoiding microvascular complications.
- Macrovascular complications affect larger blood vessels, such as those supplying the heart, brain, and extremities. The causes of these complications stem from narrowing of blood vessels due to glycation, inflammation, lipid deposition and other factors. Complications resulting from large vessel damage may lead to cardiomyopathy, stroke, rheumatoid arthritis, osteoporosis, and the degenerative process of aging (Singh et al., 2014). The major concern amongst these complications is myocardial infarction (heart attack). At present, it appears that blood glucose control does not significantly reduce the risks or delay the onset of macrovascular complications. Additional medical management is required.
Non-Pharmacological
Pharmacological
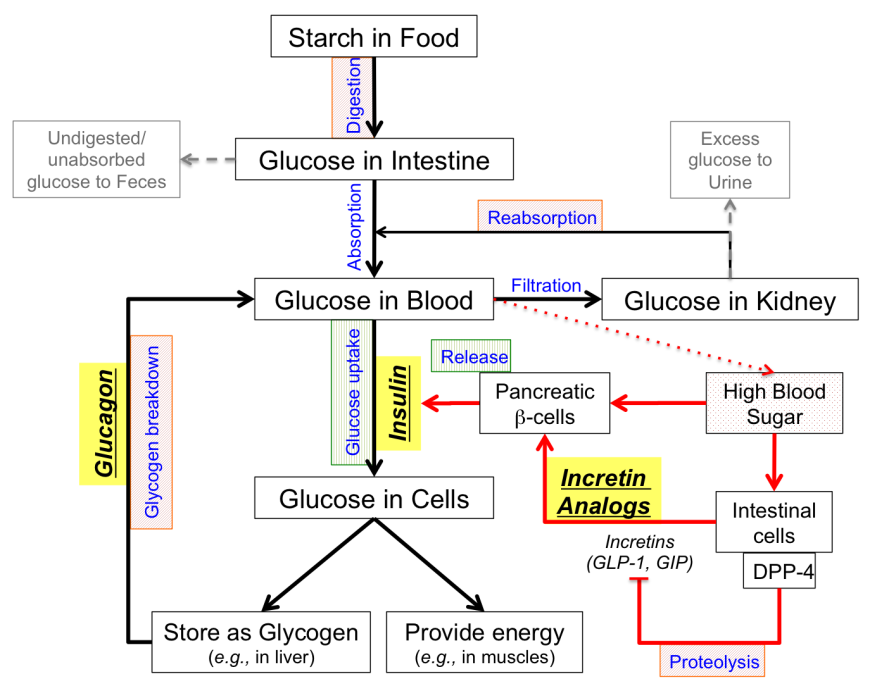
- Increasing insulin secretion may be accomplished by various classes of drugs. Some drugs increase release of insulin stored within the pancreatic β-cells, while other drugs promote insulin production and release.
- Increasing insulin function can enhance glucose uptake and utilization thereby facilitating lowering of blood glucose.
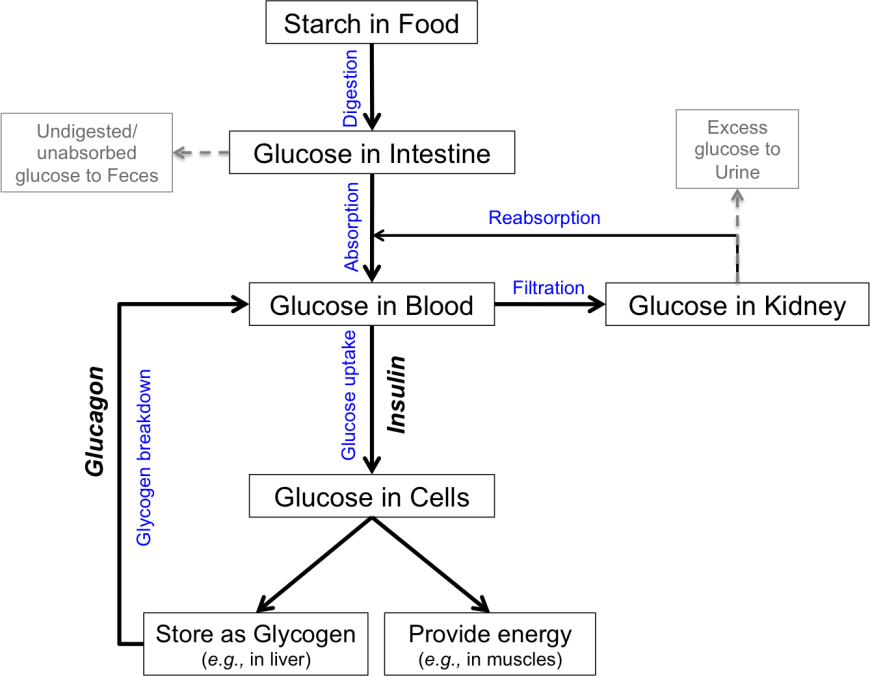
- Decreasing glucose production from liver stores of glycogen can reduce elevated blood glucose levels after overnight fasting.
- Decreasing glucose absorption from the food can be accomplished by blocking the breakdown of starch into glucose. Undigested starch is excreted in feces.
- Decreasing glucose reabsorption from the kidneys reduces blood glucose levels. Excess glucose is eliminated in urine.
| Enhancers of insulin secretion by β-cells | |
Sulfonylureas
Role(s): Enhances insulin secretion by pancreatic β-cells.
Target(s): ATP-binding cassette sub-family C member 8
Mechanism of Action: Binds to ligand-specific receptor on cytoplasmic face of pancreatic β-cells, mediating closure of ATP-sensitive K+ channel, causing depolarization, and calcium entry into cell, thereby stimulating insulin release.
Administration: Take with meals once or twice a day.
Possible side effects: Hypoglycemia.
US FDA approved drugs: Chlorpropamide (Diabinese), Glimepiride (Amaryl), Glipizide (Glucotrol), Glyburide (DiaBeta), Tolazamide (Tolinase), Tolbutamide (Orinase)
| 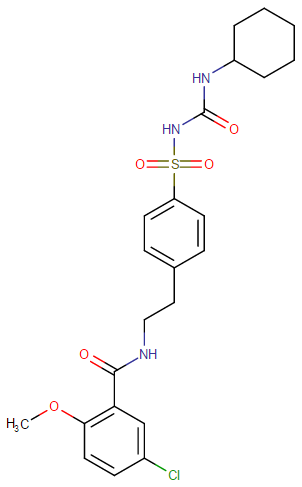
Representative Chemical Structure: Glyburide (DrugBank)
|
Meglitinides
Role(s): Enhances insulin secretion by pancreatic β-cells.
Target(s): ATP-binding cassette sub-family C member 8
Mechanism of Action: Binds to ligand-specific receptor on cytoplasmic face of pancreatic β-cells, mediating closure of ATP-sensitive K+ channel, causing depolarization, and calcium entry into cell, thereby stimulating insulin release.
Administration: Take before meals.
Possible side effects: Hypoglycemia
US FDA approved drugs: Repaglinide (Prandin), Nateglinide (Starlix)
N.B.: Meglitinides are designed to avoid hypoglycemia that may result as a side of effect of sulfonylurea administration. In comparison to sulfonylureas, they have shorter half-live and are taken at mealtimes to enhance insulin secretion and prevent postprandial (i.e., occurring after a meal) hyperglycemia.
| 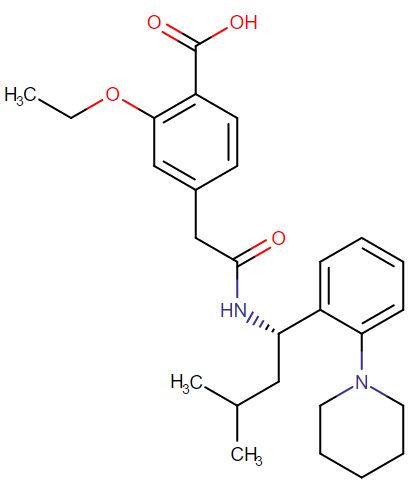
Representative Chemical Structure: Repaglinide (DrugBank)
|
Glucagon-Like Peptide-1 Analogs – peptide drugs with a longer half-life than the natural GLP-1 hormone, because they are resistant to degradation by the enzyme dipeptidyl peptidase 4 (DPP-4).
Role(s): Enhances insulin secretion by pancreatic β-cells.
Target(s): Glucagon-Like Peptide-1 receptor
Mechanism of Action: Binds to the GLP-1 receptor and stimulates insulin secretion from the pancreatic β-cells; inhibits glucagon secretion and function; delays gastric emptying into the small intestine, signals satiety and thereby suppresses food intake.
Administration: Inject peptide daily; now longer acting versions (once a week injections) are also available.
Possible side effects: May cause nausea and eventually lead to pancreatitis.
US FDA approved drugs: Liraglutide (Victoza), Exenatide (Bydureon) (injected)
| 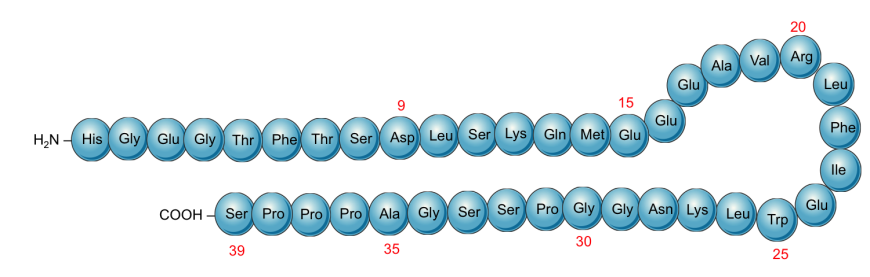
Schematic representation of the primary structure of Exenatide showing the sequence of the protein (derived from Gila monsters)
|
Dipeptidyl Peptidase-4 Inhibitors
Role(s): Enhances insulin secretion by pancreatic β-cells indirectly by prolonging the half-life or action of endogenous GLP-1.
Target(s): Dipeptidyl Peptidase-4
Mechanism of Action: Inhibits the enzyme DPP-4, resulting in increased activity of incretins (e.g., GLP-1); leads to increased insulin secretion, decreased gastric emptying, and thus lowers blood glucose levels.
Administration: Take orally once a day (at the same time each day).
Possible side effects: May lead to pancreatitis.
US FDA approved drugs: Sitagliptin (Januvia), Linagliptin (Tradjenta), Saxagliptin (Onglyza), Alogliptin (Nesina), Vildagliptin (Galvus), Anagliptin (Suiny, Beskoa), Omarigliptin (Marizev – Merck has suspended development and marketing of omarigliptin)
| 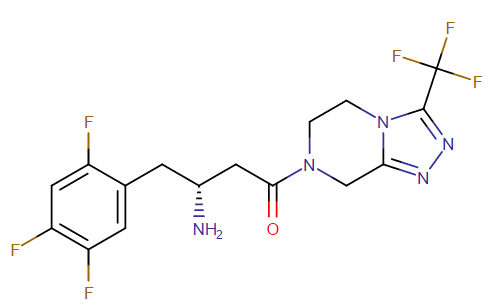
Representative Chemical Structure: Sitagliptin (DrugBank)
|
| Suppressors of Endogenous Glucose Production | |
Biguanides
Role(s): Suppresses hepatic gluconeogenesis and enhances insulin action in muscle/fat.
Target(s): 5’AMP-activated protein kinase subunit β-1
Mechanism of Action: Inhibits glucose production by the liver; increases insulin sensitivity, and stimulates glucose uptake in tissues; does not stimulate the pancreas to manufacture or secrete more insulin.
Administration: Take orally twice a day with meals.
Possible side effects: May cause gastrointestinal complications (e.g., nausea, vomiting) and risk of lactic acidosis, especially in the elderly and in those with compromised kidney function.
US FDA approved drugs: Metformin (Glucophage). Also approved in fixed dose combinations with antidiabetic drugs from other classes.
| 
Representative Chemical Structure: Metformin, the first line biguanide
|
Amylin Agonists – Synthetic analogs of the 37 amino acid peptide, amylin (islet amyloid polypeptide or IAPP), which is cosecreted with insulin by pancreatic β-cells and is synergistic in function in maintaining glycemic control.
Role(s): Suppresses hepatic gluconeogenesis.
Target(s): Amylin receptor
Mechanism of Action: Inhibits glucagon secretion and lowers preprandial rise in glucose levels; delays gastric emptying, promoting satiety, and thus inhibiting food intake.
Administration: Subcutaneous injection.
Possible side effects: Gastrointestinal complications (nausea, vomiting, abdominal pain), headache, dizziness, anorexia, fatigue and coughing.
US FDA approved drugs: Symlin (pramlintide)
N.B.: This is typically used in adjunction to insulin or metformin.
| 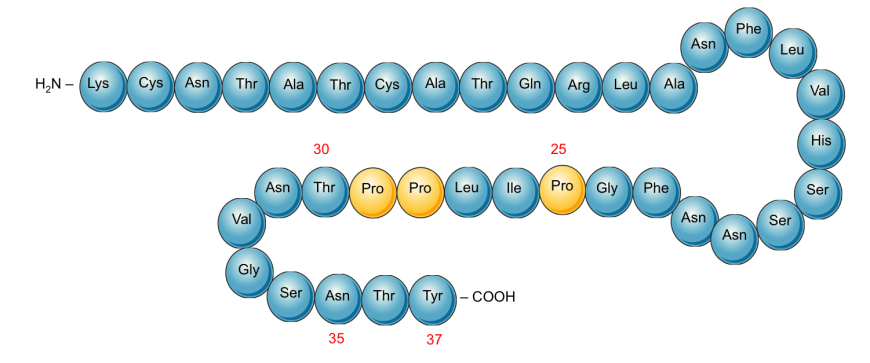
Schematic representation of the primary structure of Pramlintide. Proline substitution (changed compared to the native Amylin) at positions 25, 28, and 29 (highlighted in yellow) prevents aggregation and amyloid formation.
|
| Enhancers of insulin action in the periphery | |
Thiazolidinediones (TZDs)
Role(s): Enhances insulin sensitivity in muscle/fat cells, addressing a key problem in type 2 diabetes (i.e. insulin resistance). It also suppresses hepatic gluconeogenesis (i.e. glucose production from lipids and protein components).
Target(s): Peroxisome proliferator-activated receptor gamma (PPAR-γ)
Mechanism of Action: Acts as agonist of PPAR-γ, lowers insulin resistance and increases glucose uptake by muscle cells.
Administration: Take orally once a day at the same time.
Possible side effects: Slow onset of action, may increase risk of cardiovascular problems, fluid retention, accumulation of adipose tissue, bone loss.
US FDA approved drugs: Rosiglitazone (Avandia), Pioglitazone (Actos)
| 
Representative Chemical Structure: Rosiglitazone (DrugBank)
|
| Blockers of GI tract carbohydrate absorption | |
Alpha-glucosidase Inhibitors
Role(s): Inhibits breakdown of starches in gastrointestinal tract, indirectly reducing absorption of dietary carbohydrates.
Target(s): α-glucosidase enzymes in gut
Mechanism of Action: Works by competitively and reversibly inhibiting α-glucosidase (an enzyme responsible for degrading carbohydrates), thus slowing digestion of carbohydrates and delaying glucose absorption.
Administration: Take with first bite of meal.
Possible side effects: Gastrointestinal complications (nausea, vomiting, diarrhea).
US FDA approved drugs: Acarbose (Precose), Miglitol (Glyset: oral, not absorbed)
| 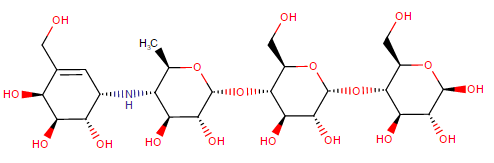
Representative Chemical Structure: Acarbose (DrugBank)
|
| Blockers of glucose reuptake in the kidney | |
Sodium-glucose transport-2 (SGLT-2) Inhibitors
Role(s): Block reabsorption of glucose from urinary tract.
Target(s): Na+/Glucose Cotransporter 2 in kidney.
Mechanism of Action: Reduces renal glucose reuptake, resulting in an increased amount of glucose excreted in urine.
Administration: Take orally once a day.
Possible side effects: Polyuria, urinary tract infections, dehydration, ketoacidosis.
US FDA approved drugs: Canagliflozin (Invokana), Dapagliflozin (Farxiga), Empagliflozin (Jardiance)
| |






0 Comments